
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
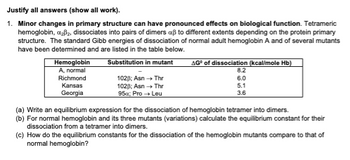
Transcribed Image Text:Justify all answers (show all work).
1. Minor changes in primary structure can have pronounced effects on biological function. Tetrameric
hemoglobin, a₂³₂, dissociates into pairs of dimers aß to different extents depending on the protein primary
structure. The standard Gibb energies of dissociation of normal adult hemoglobin A and of several mutants
have been determined and are listed in the table below.
Substitution in mutant
Hemoglobin
A, normal
Richmond
Kansas
Georgia
102B; Asn → Thr
102B; Asn → Thr
95x; Pro → Leu
AG® of dissociation (kcal/mole Hb)
8.2
6.0
65
5.1
3.6
(a) Write an equilibrium expression for the dissociation of hemoglobin tetramer into dimers.
(b) For normal hemoglobin and its three mutants (variations) calculate the equilibrium constant for their
dissociation from a tetramer into dimers.
(c) How do the equilibrium constants for the dissociation of the hemoglobin mutants compare to that of
normal hemoglobin?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps

Knowledge Booster
Similar questions
- 5. Anesthetic gases used in surgery are known to bind to the hemoglobin molecule in red blood cells. The diagram below illustrates O2 binding curves of normal human HbA in the presence of the anesthetic gas dichloromethane (DCM). O UNTREATED 100 a Dсм 23 Torr Symbols: в Dсм 50 Torr x DCM I00 Torr o, 0 Torr DCM; O, 23 Torr DCM A, 50 Torr DCM x, 100 Torr DCM. 80 60 40 (1 Torr = 1 mm Hg.) 20 The solutions were buffered to pH 7.4. 0.5 1.0 1.5 2.0 log p02 (a) (* Hill plots. Label the axes and indicate on both the plot above and the Hill plot where the value of the dissociation equilibrium constant Ka for O2 binding is defined for 0 and 100 Torr of dichloromethane For the curves at 0 and 100 Torr of dichloromethane, draw their equivalent in the form of Kd Hb(O2)n Hb + nO2 % OXYGENATIONarrow_forwardCan you check if A is correct and please help answer the following questions?arrow_forwardYou have discovered a new hemoglobin variant you are calling Hb-21. You have noticed the following from your studies of Hb-21: - The binding constant of Hb-21 is the same at pH 7.1 and 7.4.- The binding constant curves generated by Hb-21 and O2 are sigmoidal.- The Hill coefficient for Hb-21 is 3.-The binding constant for Hb-21 with O2 in the presence of 5mM 2,3-bisphosphglycerate is about 6-times lower instead of the 8-times lower that is observed under these conditions for the wild type enzyme. Use this information to answer questions: Based on this information which of these features of hemoglobin would you conclude are altered in Hb-21 as compared to the wild type hemoglobin? ____ the cooperativity.____ the amount of T versus R state in the tissues. ____ the Bohr effect. What is the major issue for an individual with the Hb-21 variant? Explain exactly what aspect of hemoglobin’s function is altered.arrow_forward
- 2. (6) Examine the two proteins labelled a) and b). Which of the two Ramachandran plots, c) or d) is more likely to be derived from which protein? Explain how you determined this. (a) (b) (c) (d) ✓ (degrees) ✓ (degrees) +-180 120 60 -60 -120 -180 -180 +180 120 60 -120 -180 -180 0 (degrees) 0 (degrees) +180 +180arrow_forwardList 4 different actiated carrier molecules, for each one, list both the oxided and reduced formsarrow_forward10. A new protein of unknown structure has been purified. Gel filtration chromatography reveals that the physiological protein has a molecular weight of 240,000 Daltons. Chromatography in the presence of 6 M urea yields a single peak corresponding to a protein of 60,000 Daltons. Chromatography in the presence of 6 M urea and 10 mM mercaptoethanol yields peaks for proteins of 34,000 Daltons and 26,000 Daltons. What can we learn about protein's tertiary and quaternary structure from this data?arrow_forward
- Why does the dissociation constant change for hemoglobin in high and low 02 concentrations? O Hemoglobin is a tetramer and subunits have decreased dissociation of 02 when a neighboring subunit has bound 02 O Hemoglobin is a monomeric protein with increased dissociation of 02 when a neighboring molecule has bound 02 O Hemoglobin is a tetramer and subunits have increased dissociation of 02 when a neighboring subunit has bound CO O Hemoglobin is a tetramer and subunits have increased dissociation of O2 when a neighboring subunit has bound O2arrow_forward4)arrow_forward5. Anesthetic gases used in surgery are known to bind to the hemoglobin molecule in red blood cells. The diagram below illustrates O2 binding curves of normal human HbA in the presence of the anesthetic gas dichloromethane (DCM). O UNTREATED 100 Symbols: a Dсм 23 Torr • DCM 50 Torr x DCM I00 Torr o, 0 Torr DCM; О, 23 Torr DСМ Д, 50 Torr DСМ x, 100 Torr DCM. 80 60 40 (1 Torr = 1 mm Hg.) 20 The solutions were buffered to pH 7.4. 0.5 1.0 1.5 2.0 log p02 For the curves at 0 and 100 Torr of dichloromethane, draw their equivalent in the form of (a) (* Hill plots. Label the axes and indicate on both the plot above and the Hill plot where the value of the dissociation equilibrium constant Kd for O2 binding is defined for 0 and 100 Torr of dichloromethane Kd Hb(O2)n nO2 Hb + Be sure to label the axes, indicate the units on both axes, and make the Hill plot so that the values of Kd are equivalent to those on the saturation plot above. Indicate whether cooperativity, as indicated by the Hill…arrow_forward
- 20. The dissociation constant of protein Z for ligand Y is: 10 micromolar. What fraction of the ligand is bound at ligand concentration = 5 micromolar?arrow_forwardThe T state of hemoglobin is converted to the R state by what event? Select one: The binding of oxygen destabilizes a more planar heme ring which alters the position of the proximal histidine and subsequently, residues between the alß2 interface. а. b. None of these. The binding of oxygen stabilizes a more planar heme ring which alters the position of the proximal proline and subsequently, residues between the alß2 interface. С. d. The binding of oxygen stabilizes a more planar heme ring which alters the position of the proximal histidine and subsequently, residues between the alß2 interface. The binding of oxygen destabilizes a more planar heme ring which alters the position of the proximal proline and subsequently, residues between the alß2 interface. е.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON