
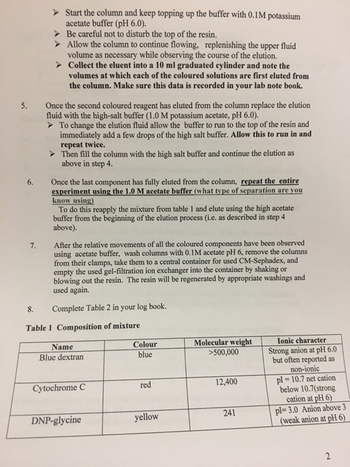
1. Know the structures of DNP-glycine and of the chromophore associated with cytochrome C.
Low Salt
Volume of blue band = 5.2 ml (Void Volume)
Volume of yellow band = 2.6ml
Volume of red band = 11ml
High Salt
Volume of blue band = 4.2 ml -> Void Volume
Volume of yellow band = 5.2
Volume of red band = 1.2
2. What is the exclusion limit and the fractionation range for CM-Sephadex resin used?
3. What are the ion exchange reactions which occur when the coloured mixture is first applied to the column under low salt conditions? What exchange reactions occur upon the change of buffer from low salt to high salt concentrations?
4. What were the values for Vo and Vi for this column? Where the values for Vo and Vi the same under both conditions?
5. Explain the order in which the various components of the mixture elute under each condition in terms of their physical properties and the nature of the CMSephadex G-50 column. Would the same order be observed with a G-50 Sephadex column (i.e. no CM component)?
6. Predict and explain the results that would have occurred if the same experiment had been run with a CM-Sephadex G-50 column equilibrated in and eluted with 0.1 M HCl or 1M HCl
7. Predict movement of protein hemoglobin: heme containing protein, 64,500 Daltons, reddish brown, pI=6.9 or vitamin B12: non-protein organic molecule, 1,355 Daltons, pink, pI=6-8 in the above two columns with blue dextrose, cytochrome c and DNP-glycine. If attempting this separation would you attempt to use more than two salt concentrations? Why? Would a different resin or cutoff be more appropriate.

Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

- 10. In an abnormal hemoglobin variant known as Hemoglobin-Kariya, the lysine residues at position 40 in the ß-chains are replaced by glutamic acid residues. a) What effect would you expect this change to have on the quaternary structure and oxygen binding properties of hemoglobin? b) Draw a diagram to illustrate the difference in the oxygen binding curves that would be obtained with normal hemoglobin and Hemoglobin-Kariya.arrow_forward22. Describe the Induced fit of Hemoglobin, Hb (enzyme-like protein) which has quarternary structure and exhibits allosteric activation with cooperativity.arrow_forward6. Consider the following proteins to answer the questions below: Protein Size (kDa) pl ε at 280 nm 10 4 7000 50 4 14000 10 8 3000 50 8 50000 A B C C Red Colored? Yes No No No b. Describe a two-step purification procedure that could be used to purify/isolate protein A from the other proteins. In your response, describe the type of chromatography used, the pH of buffer needed, and a labeled chromatogram (include absorbances at both 280 and 400 nm). Make sure you note which "fraction/sample" is needed from the first step to proceed/use for the second step. Use another page if necessary.arrow_forward
- 1. A variety of organic chelating ligands have been synthesized to tightly coordinate radioactive metal cations to identify and treat malig- nancies by coupling the metal complex to a polypeptide linker attached to a monoclonal antibody that binds specifically to a cell surface re- ceptor. One such metal complex is illustrated in the diagram on the right. The radioactive Cu-64 cation is tightly coordinated by the che- lating ligand that is, in turn, conjugated to a peptide linker attached to a monoclonal antibody. (The antibody is not shown in this diagram.) When coupled to a specifically designed monoclonal antibody, the complex binds specifically to somatostatin receptors that are ex- pressed on the surface of neuroendocrine tumors. Subsequently the entire complex with the receptor is internalized, i.e., passed into the cytoplasm, where the radioactive metal cation kills the malignant cell. CH3 HN NHNH HN NHNH HN НО НО NH HN HN HO S-S NH (a) dues in the polypeptide. Place the label…arrow_forward2. The following tyrosine-phosphorylated polypeptides were tested as substrates of protein tyrosine phosphatase, an important enzyme in cellular signal transduction. The pTyr in bold signifies the tyrosine residue that is phosphorylated. The binding affinities of the polypeptide substrates to the enzyme were found to follow the order: (a) > (c) - (d) >> (b). (a) Asp-Ala-Asp-Glu-pTyr-Leu-lle-Pro-Gln-Gln-Gly (b) Pro-Val-Pro-Glu-pTyr-lle-Asn-Ala-Ser-Val (c) Asp-Ala-Glu-Glu-pTyr-Leu-Val-Pro-Gln-Gln-Gly (d) Asp-Asn-Leu-Tyr-pTyr-Trp-Asp-Glu-Asn-Ser-Ser In addition to the presence of the phosphorylated tyrosine residue, what amino acid a) properties of the polypeptides accounts for the relative order of binding affinities to the enzyme? b) the active site of the phosphatase enzyme that are responsible for the affinity of substrate binding? Assume a pH of 7.4 at the enzyme active site. (Note a phosphatase uses phosphate substrate) What amino acids have side chains that would indicate they are…arrow_forwardE Threonine 6. You have identified some intermediates in threonine synthesis: A, B, C, D and E. You grow a few of your mutants in the presence of these different intermediates to determine the order in which the gene products act. Below are your results. A (+) means growth and a (-) means no growth. Given these data, draw the best possible pathway for the synthesis of threonine. The diagram should use arrows to indicate one intermediate being changed to another intermediate. Indicate which gene produces the product responsible for the conversion by listing the mutant in that gene above the arrow. Mt1 Mt2 Mt4 Mt7arrow_forward
- 25. Assume we are performing a 2x serial dilution series of methylene similar to WL-1. If your original, 1x tube is designated "Tube 1", then what would be the relative concentration of methylene blue in Tube 4? a. Tube 4 is the 8x dilution tube. The concentration of methylene blue in this tube is 1/8 that of the original tube. b. Tube 4 is the 8x dilution tube. The concentration of methylene blue in this tube is 8 times that of the original tube. c. Tube 4 is the 4x dilution tube. The concentration of methylene blue in this tube is 4 times that of the original tube. d. Tube 4 is the dilution 10x tube. The concentration of methylene blue in this tube is 1/10 that of the original tube. e. None of the abovearrow_forward7. [10'] For a bacteriophage 77, the following data at zero concentration have been obtained: Sedimentation coefficient: $20,w = 453 S. Diffusion coefficient: D₂0. = 6.03×10-8 cm² s¹¹. 20,w Specific volume V20 = 0.639 cm³ g¹ Calculate the molecular mass M of the bacteriophage.arrow_forwardVII. Analysis of a peptide antibiotic purified from a strain of Bacillus brevii resulted in the following significant information: Complete hydrolysis of the peptide yielded equimolar amounts of Leu, Orn, Phe, Pro, and Val Molecular weight of the peptide was estimated to be aroung 1,200 Da The peptide failed to undergo hydrolysis when treated with carboxypeptidase Partial hydrolysis and then chromatographic separation of products yielded the following di- and tripeptides: Leu-Phe, Phe-Pro, Orn-Leu, Val-Orn, Val-Orn-Leu, Phe-Pro-Val, Pro-Val-Orn Treament with dinitrofluorobenzene (DNFB) followed by complete hydrolysis yielded only free amino acids and the DNFB-derivatized ornithine at its side chain Using this set of information available to you, deduce the amino acid sequence of this peptide antibiotic. Write your final peptide sequence only using 3-letter abbreviations.arrow_forward
- 2. The diagram to the right shows the change in the structure of the C-terminal portion of each of the ẞ-subu- nits of human hemoglobin (HbA) in the oxyHb to deox- yHb or R-to-T transition. The hydrogen bonding interac- tion of the C-terminal ẞHis 146 residue with the side chain of Asp94, highlighted by the red ellipse, has been shown to be responsible for a major portion of the proton uptake associated with the Bohr effect. Treatment of HbA with the enzyme carboxypeptidase A (CPA) results in loss of the C-terminal ẞHis 146 and ẞTyr145 residues of the ẞ- subunits. (a) ( ) Draw a Hill plot [log(Y/[1-Y]) vs. log(pO2), Y = fraction of heme groups occupied by O2] to compare the values of the Hill coefficient nн and the O2-binding affinity at pH 7.4 of normal HbA before and after treat- ment of with CPA. (b) (' ) How will the plot for CPA-digested HbA change at pH 7.2? (c) 1 Hi5146 HN- ✓ Low PK B-chain. CH2 CH-NH-CO-CH-NH- CH₂ Tyr145 он HbO2 or R state Туг145 CH-CH2- OH co NH CH CH₂ His…arrow_forwardVI. Although most biologically relevant sugars have the D-configuration, L-fucose is a commonly observed monosaccharide component in glycoproteins. Glycosylated antibodies (IgGs) observed in patients with active TB have more fucose than those found in patients with latent TB (Lu et. al., 2016). Latent TB occurs when a person has the TB bacteria, but bacterial proliferation is kept under control by the body's immune system; hence, the infected person is asymptomatic and not infectious. A person with latent TB will test positive for the tuberculin test or TB blood test. a) L-fucose is also known as 6-deoxy-L-galactose. Note that D-galactose is a C-4 epimer of D-glucose. Draw the Fisher projections for D-glucose, D-galactose and L-fucose. (Hints: D- and L-isomers are enantiomers. 6-deoxy means that the hydroxyl group at C-6 is removed leaving just the methyl group). D-galactose: D-glucose: L-fucose (6-deoxy-L-galactose): b) L-fucose can cyclize into a pyranose and form two anomers. Draw…arrow_forward5. Anesthetic gases used in surgery are known to bind to the hemoglobin molecule in red blood cells. The diagram below illustrates O2 binding curves of normal human HbA in the presence of the anesthetic gas dichloromethane (DCM). O UNTREATED 100 a Dсм 23 Torr Symbols: в Dсм 50 Torr x DCM I00 Torr o, 0 Torr DCM; O, 23 Torr DCM A, 50 Torr DCM x, 100 Torr DCM. 80 60 40 (1 Torr = 1 mm Hg.) 20 The solutions were buffered to pH 7.4. 0.5 1.0 1.5 2.0 log p02 (a) (* Hill plots. Label the axes and indicate on both the plot above and the Hill plot where the value of the dissociation equilibrium constant Ka for O2 binding is defined for 0 and 100 Torr of dichloromethane For the curves at 0 and 100 Torr of dichloromethane, draw their equivalent in the form of Kd Hb(O2)n Hb + nO2 % OXYGENATIONarrow_forward
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON





