
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
Please answer legibly.
What is the meaning of these equations: Langmuir equation and Freundlich equation
Please give the meaning of each variable in those two equations.
For example: Beer-Lambert Law.
A= ϵbC A = absorbance; ϵ = molar absorptivity; b = length of light path; c = concentration. This Beer-Lambert law is just an example of how to do it with the two equations: Langmuir and Freundlich (attached pictures)
Also, please include it in the nomenclature.
Nomenclature - The symbols should be defined in the nomenclature in alphabetical order. The accompanying definitions must include proper units.
|
Symbol |
Meaning |
Units |
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
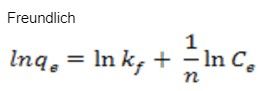
Transcribed Image Text:Freundlich
1
Inq, = Ink, + = In C₂
kf
n
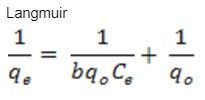
Transcribed Image Text:Langmuir
1
96
=
1
bq. Ce
с
+
1
90
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Similar questions
- What is the Gibbs phase rule for the general system?arrow_forwardderivation of ideal gas equation for kinetic theory of gas 1.frequency of collision 2.derive the force 3.To determine the pressure of the gas 4.to determine energy associated with ideal gas 5.determine ideal gas equation 6.determine V_rms² from ideal gas Please break each derivation step by step for better understanding ,if possible include diagramarrow_forwardQUESTION 1 Match the following terms to the correct definition. Sublimation Dipole-dipole attraction Adhesive force Viscosity Intermolecular force Dispersion force Surface tension Phase diagram A. force of attraction between molecules of different chemical identities B. "noncovalent attractive force between atoms, molecules, and/or ions" C. intermolecular attraction between two permanent dipoles D. "energy required to increase the area, or length, of a liquid surface by a given amount" E. "attraction between two rapidly fluctuating, temporary dipoles; significant only when particles are very close together" F. measure of a liquid s resistance to flow G. pressure-temperature graph summarizing conditions under which the phases of a substance can exist H. change from solid state directly to gaseous statearrow_forward
- In a 2-component, 2-phase system, write Raoult's law for component 1 and component 2, and derive the equation that relates Bubble temperature to Bubble point, and the equation that relates Dew temperature to Dew point.arrow_forwardIf a dispersion of particles had zero charge (as measured by their zeta potential) and no surfactant coating, what would you expect dynamic light scattering to show in terms of effective hydrodynamic particle radius? A small negative value A value of zero A small positive value A large positive value A bimodal distribution of small and large value 25arrow_forwardA question in chemical engineering.arrow_forward
- (1) For the cubit unit cell shown below, a. How many atoms are there within in the cell? b. What is the name of the lattice characterized by this unit cell? Explain your answer.arrow_forwardWhich of the following statements is correct regarding TCD detector?arrow_forward1. A sunphotometer is an instrument that looks at visible light coming in a straight line directly from the sun with F0 = 1850 W/m²/um. At one point with cloudy sky and aerosol plume under the cloud, it measures an irradiance of I1 W/m²/um. When the cloud passes this increases to 12 W/m²/um and when there were no aerosols, the irradiance became 13 W/m²/um. Assuming sun zenith angle 0 degrees: a) What is the optical depth of the cloud? b) If the cloud would be twice thinner, what intensity would the sunphotometer measures when the cloud was overhead? What is the transmittance for this case? c) What are the optical thicknesses of aerosol and Rayleigh scattering components?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The