
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
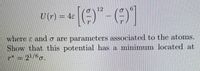
Transcribed Image Text:12
U (r) = 45
where ɛ and o are parameters associated to the atoms.
Show that this potential has a minimum located at
p* = 21/6g.
Expert Solution
arrow_forward
Step 1
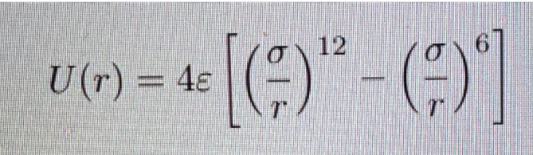
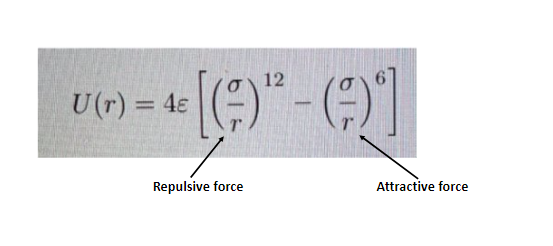
The above mentioned equation is known as Lennard jones potential equation.
The potential U(r) is a combination of repulsive and attractive forces.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- Given: gE/RT = 4 x1x22 Determine the expression for the activity coefficient for component 1. Then, evaluate γ1∞.arrow_forwardA question in chemical engineering.arrow_forwardThe second virial coefficient is related to pair-wise potential function via B(T) = 2πNA (1- e-/kr) r²dr. Use the hard sphere pairwise potential function to find B(T).arrow_forward
- Compute the fugacity and chemical potential for CO2 at 323 K and 5 bar. At these conditions, CO2 is described by the virial equation of state truncated at the second virial coefficient. Assume that the interaction potential between CO2 molecules can be described by the spherically symmetric square-well potential.arrow_forwardHelp with the following question. Which of the temperature scales in common use can be used directly in the Stefan-Boltzmann law? arrow_forwardHow many intensive variables are necessary to completely specify the state of pure water and pure glycerol at temperatures above 18 degrees C. What are they? If the pressure is fixed at 1 bar, how many intensive variables are necessary?arrow_forward
- Sketch very roughly the phase diagrams for water and carbon dioxide and use them to answer this question: at a pressure of X atmospheres and a temperature Y degrees Kelvin,what is the phase of water, and what is the phase of carbon dioxide Y=9.43 X=943arrow_forwardE Open with Google Docs OCESSES OF PURE SUBSTANCES.pdf EXAMPLES A closed rigid vessel having a volume of 100 m3 contains 10 m3 of saturated liquid water and 90 m3 of saturated water vapor at 1.0 MPaa. Heat is transferred until the vessel filled with saturated vapor. Determine the heat transfer.arrow_forwardA mixture of three components (A, B, and C) enters a separation process.The three components appear in the distillate with variable composi-tion. In contrast, only B and C appear in the bottom. Write a proper set ofmaterial balance equationarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The