
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
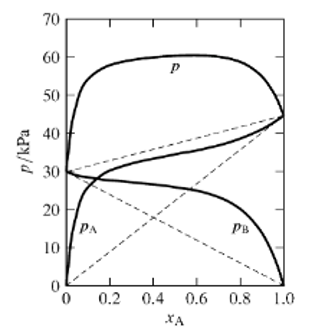
1- what if B = benzene, A = methanol, what are the dominant interactions between A and B, B and B and A and A and what is their dependence on the distance of the molecules? What can you follow about the strength of these interactions from the phase diagram?
2- Indicate in the figure which limiting cases you can identify. Name the limiting cases and write down the equations for it. Explain in which situations they hold.
3- You set up a solution of 70 mole % A and 30 mole % B. Calculate from the diagram of A in the gas phase. What does this indicate for a possible separation of A and B and how could you get that?
Transcribed Image Text:p/kPa
70
60
50
40
30
20
10
0
PA
PB
0.2 0.4 0.6 0.8 1.0
XA
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 6 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- In an experiment, a 0.001 (mole fraction) solution of polysaccharide in water is made and is placed in the compartment A (see Figure below). Compartment B is filled with pure water. The two compartments are separated by a porous semi-permeable membrane that allows the exchange of water molecules between the two compartments, but not that of the larger polysaccharide molecules Part 2: As a result of this chemical potential difference, water molecules will move from compartment B to compartment A. This causes the pressure in compartment A, relative to that in B, to increase. How would this affect the chemical potential of water in compartment B? When would the diffusion of water from B to A cease (i.e. equilibrium is achieved)?arrow_forward1. The following data was obtained for liquid A and B which is partially miscible. T (K) 309.82 309.42 309.03 308.00 306.68 0.400 0.371 0.473 0.527 0.600 0.629 0.674 0.761 304.55 301.80 299.09 0.326 0.239 0.255 0.745 XA XB 296 0.218 0.193 0.168 0.782 0.807 0.832 294.53 0.157 0.843 (a) Plot the phase diagram of liquid A and B (b) State the composition of two phases that form from mixing 0.750 mol of A and 0.250 of B at 300 K. (c) To what temperature must the mixture be heated to form a single-phase mixture?arrow_forwardExplain using an example the relationship of chemical potential to phase equilibrium.arrow_forward
- On a single-component phase diagram, one must cross a coexistence line to move from the region for liquids to the region for gases. True Falsearrow_forwardPlease solve this questionnnnnn correctly, question please, please UR solutionarrow_forwardIn this lab, you will start off by calculating the number of moles of air molecules inside a flask containing 20 mL of water by cooling the flask down to near 0°C and assuming the vapour pressure of water is negligible at that temperature. If the volume of the air inside the flask is 145.2 mL, the gas pressure sensors reads 101.3 kPa and the temperature sensor reads 3.7 °C, how many moles of air are present in the flask? Express your answer in mol. Assume R = 8.314 J mol-¹ K-¹. Answer:arrow_forward
- Raisin In D Shagun x C Clever | P X SCHEMIST x N Chemistr X O Microsof x Schoolog X OAtoms |x A districtims.seattleschools.org/common-assessment-delivery/start/4777996531?action=Donresume&submissionld D464527652 Drag the pictures into the table below. Place them in order from weakest attractive force between the proton(s) and electron to strongest attractive force between the proton(s) and electron weakest attractive force strongest attractive force 0.20 nm 0.10 m 0.10 nm 1arrow_forward3.arrow_forward4) For such a phase diagram, a) Do you expect the newly formed bonds (A-B) are stronger than the initial bonds or not? Briefly explain the reason. Is this solution formation reaction endothermic or exothermic? b) Then, is the activity coefficient greater than 1 or not? How would you expect change in volume and change in Enthalpy due to formation of the solution? c) Describe the function (interatomic bond parameter). Explain/compare the bond strength (newly formed A-B vs initial A- A and B-B) depending on sign of the 2-function. T L+α L A Хв Barrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY