
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
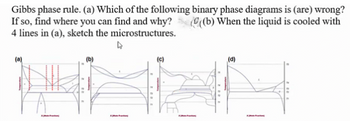
Transcribed Image Text:Gibbs phase rule. (a) Which of the following binary phase diagrams is (are) wrong?
If so, find where you can find and why? (((b) When the liquid is cooled with
4 lines in (a), sketch the microstructures.
↳

Transcribed Image Text:4. The number of degrees of freedom (F) which a system containing C components
can have when P phases are in equilibrium is given by F= C-P+2, called as the
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Using the Gibbs phase rule determine the number of components, number of phases and hence the number of degree of freedom using the Gibbs phase rule for each of the following systems: An aqueous solution containing Na+, C?-, K+ and Br- ions.arrow_forwardthe repulsive Suppose that a gas obeys the van der Waals equation of state With much greater that the attractive effects. Calculate the change in molar Gibbs energy when the pressure is changed from pi to pf isothermally. Given: b = 4.566x10-5 m³/mol, R = 8.3145 J/K/mol, T = 300 K, P₁ = 1.478 bar, Pf = 3.578 bar. AGM = J/mol. 4 sig. fig.arrow_forwardA sample of water vapour at 200 °C is compressed isothermally from 288 cm³ to 67 cm³. What is the change in its molar Gibbs energy? J/mol. 4 sig. figures.arrow_forward
- 1. A certain amount of NH4Cl(s) was put into a vacuum container, and then it is heated until equilibrium was reached: NH4Cl(s) == NH3(g) + HCl (g). Please write the number of phase p, the number of component C and the number of freedom F.arrow_forwardSuppose that in a phase diagram, when the sample was prepared with the mole fraction of component A equal to 0.40 it was found that the compositions of the two phases in equilibrium corresponded to the mole fractions xA,α = 0.60 and xA,β = 0.20. What is the ratio of amounts of the two phases?arrow_forwardOn a single-component phase diagram, one must cross a coexistence line to move from the region for liquids to the region for gases. True Falsearrow_forward
- Please solve this questionnnnnn correctly, question please, please UR solutionarrow_forwardGive detailed Solution asaparrow_forwardSuppose now that argon is added to the mixture in the previous exercise to bring the composition closer to real air, with mole fractions 0.780. 0.210, and 0.0096, respectively. (a) What is the additional change in molar Gibbs energy and entropy at 298 K? (b) Is the mixing spontaneous?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY