
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
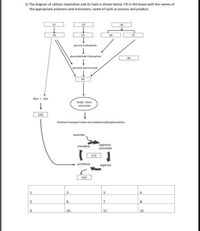
Transcribed Image Text:1) The diagram of cellular respiration and its fuels is shown below. Fill in the boxes with the names of
the appropriate polymers and monomers, name of cycle or process and product.
(1)
(2)
(3)
(4)
(5)
(6)
(7)
glucose-6-phopshate
elyceraldehyde-3-phosphate
(8)
pyruvate (pyruvic acid)
(9)
NH) + CO2
Krebs Citric
acid cycle
(10)
Electron transport chain and oxidative phosphorylation
aspartate
arginino-
succinate
citrulline
(11)
ornithine
arginine
(12)
1.
2.
3.
4.
6.
7.
8.
9.
10..
11.
12.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Similar questions
- We eat foods containing sucrose (table sugar), lactose (milk sugar), and cellobiose (disaccharide of cellulose in plants). How many net moles of ATP per mole disaccharide do humans generate from the disaccharides sucrose, lactose, and cellobiose using the glycolytic pathway? Osucrose: 4 ATP; lactose: 2 ATP; cellobiose: 2 ATP Osucrose: 2 ATP; lactose: 4 ATP; cellobiose: 2 ATP Osucrose: 2 ATP; lactose: 2 ATP; cellobiose: 0 ATP sucrose: 6 ATP; lactose: 2 ATP; cellobiose: 0 ATP sucrose: 4 ATP; lactose: 4 ATP; cellobiose: 0 ATParrow_forwardThe cofactor shown below: is an oxidizing agent is a reducing agent is a carrier of acyl groups is flavin mononucleotide The cofactor shown below: CH2 HC OH HC OH HC OH CH2 H₂C NH H H H OH OH NH₂ 1) is an oxidizing agent 2) is a reducing agent 3) is a carrier of acyl groups 4) is flavin mononucleotidearrow_forward1 of 1 3. A sample of glucose reacts in anaerobic respiration. The right- hand box below shows a particle diagram of the moles of substances present after the reaction is complete. fe On a piece of paper draw the "Before" box as shown and draw a particle diagram of the reactant molecules that produced the mixture shown on the right. Key = C2H5OH = CO2 = CgH1206 Sub Before After You will need to draw a diagram to answer this question. On a piece of paper, draw the "Before" box as shown, and then draw a particle diagram of the reactant molecules that produced the mixture shown on the right. Upload an image of your drawing by clicking "Upload files" or by dragging and dropping your file into the box. Or, use your device's camera to take a photo of your work by clicking the camera icon.arrow_forward
- Although the first two carbons of fructose and glucose are identical in structure to DHAP and GADP (from glycolysis), DHAP and GADP equilibriate on their in solution to favor the ketone over the aldehyde, while fructose and glucose do not. Why? a)The larger size of the molecule sterically hinders the isomerization b)The larger sugars have more OH groups which hydrogen bond and disrupt isomerization c)The larger sugars cyclize, and there is no carbonyl to isomerize in the cyclic form d)The larger sugars cyclize, and in the cyclic form the hydrogen bonding is very strong e)The larger sugars are less soluble in water than the smaller sugarsarrow_forwardSelect lipid samples under triacylglycerol (one simple and one mixed), phospholipid, and cholesterol-derived metabolite. Show the structure and briefly describe the function or significance of each.arrow_forwardPlease show all your work please, thank you! Sorry, this question has many parts. The reaction O3(g)+O(g)→2O2(g), occurs via the following mechanism:Step 1: Cl(g)+ O3(g)→ClO(g)+O2(g); Ea=85 kJ/molStep 2: ClO(g)+ O(g)⇌Cl(g)+O2(g); Ea=10 kJ/mol a. Given the proposed mechanism above, identify any catalysts and any intermediates in this reaction. Be sure to distinguish between the two types of species in your answer. b. Using the proposed mechanism, derive a rate law. *Hint: use the activation energies to determine the slower step. Show all of your work. c. This mechanism was verified by kinetic measurements of the rate of depletion of ozone. How is the rate of change of oxygen gas concentration related to the rate of change of ozone concentration?arrow_forward
- 8. Write the systematic name for each glycosidic bond. CH₂O HO OH CH OHHO 8-439 OH HO OH CHION QIL CH₂OH tollarrow_forwardWhich of the compounds shown below exhibit a high negative free energy of hydrolysis equal to or larger than the free energy of hydrolysis for the phosphoanhydride of ATP? (Choose all that apply) В Α HPΗ Ho HO HO H2N-CH-C CH2 H2N-CH-C CH2 CH2 H2N-CH-Ö CH2 C CH2 D E O A O B O E O=p-00 O=p-00 O=-00 O=0-00 O=L-00arrow_forwardIf the glucose molecule was labeled with 14C at the C-3, which carbon atom in pyruvate would you expect to find labeled? Select the structure of pyruvate showing its appropriate structure at pH 7.4. The structure should have a carbon highlighted in green and bold that corresponds to the original 14C labeled C-3 of glucose. H3C-C-C-OH H3C-C-C -O H3C-C-c-OH H3C C-C- H3C-C-C- H3C-C HO-arrow_forward
- Name the types of glycosidic bonds found in this olygomer, from left to right (from 1 to 3) CH 20H CH 2OH CH 2OH но OH OH OH OH OH OH O alpha1-1, alpha1-4, n O alpha1-4, alpha1-4, n O alpha1-4, beta1-2 O alpha1-4, beta1-4 O beta1-4, alpha 1-4 « Previous Next Not saved Submit Quizarrow_forwardDraw the chemical structure of ATP 1. Circle and label the following parts: Adenine, Ribose, Tri-Phosphate. 2. Put a bracket around and label the part that is called Adenosine. 3. Use nested brackets and labels to identify AMP, ADP and ATP 4. Use arrows to identify the high energy bonds.arrow_forwardC Phosphoglycerate enolase coo O H-C-O-P=O CH₂OH 2-Phosphoglycerate This is a (an) d Tyrosine hydroxylase HO coo NH3 L-Tyrosine This is a (an) 0₂ coo C-O-P-C || CH₂ Phosphoenolpyruvate H₂O + HO HO L-DOPA H₂O coo NH3arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON