
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
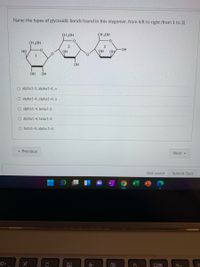
Transcribed Image Text:Name the types of glycosidic bonds found in this olygomer, from left to right (from 1 to 3)
CH 20H
CH 2OH
CH 2OH
но
OH
OH
OH
OH
OH
OH
O alpha1-1, alpha1-4, n
O alpha1-4, alpha1-4, n
O alpha1-4, beta1-2
O alpha1-4, beta1-4
O beta1-4, alpha 1-4
« Previous
Next
Not saved
Submit Quiz
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps

Knowledge Booster
Similar questions
- The cofactor shown below: is an oxidizing agent is a reducing agent is a carrier of acyl groups is flavin mononucleotide The cofactor shown below: CH2 HC OH HC OH HC OH CH2 H₂C NH H H H OH OH NH₂ 1) is an oxidizing agent 2) is a reducing agent 3) is a carrier of acyl groups 4) is flavin mononucleotidearrow_forwardGive the omega-n designation for each acid. Part 1 of 2 stearidonic acid CH3CH2(CH=CHCH2) 4 (CH2)2COOH omega- acid Part 2 of 2 linoleic acid CH3(CH2) CH=CHCH2CH=CH(CH2), COOH omega- acidarrow_forwardAlthough the first two carbons of fructose and glucose are identical in structure to DHAP and GADP (from glycolysis), DHAP and GADP equilibriate on their in solution to favor the ketone over the aldehyde, while fructose and glucose do not. Why? a)The larger size of the molecule sterically hinders the isomerization b)The larger sugars have more OH groups which hydrogen bond and disrupt isomerization c)The larger sugars cyclize, and there is no carbonyl to isomerize in the cyclic form d)The larger sugars cyclize, and in the cyclic form the hydrogen bonding is very strong e)The larger sugars are less soluble in water than the smaller sugarsarrow_forward
- 8. Write the systematic name for each glycosidic bond. CH₂O HO OH CH OHHO 8-439 OH HO OH CHION QIL CH₂OH tollarrow_forwardExplain the structure and its componentsarrow_forward.1 Given structures (Betaxlol) and (Timolol), which one would be a better choice topically to treat glaucoma in a patient with a history of asthma? Provide a SBTE for your answer. cr 0- CH2-CH-CH2-NH2-CH(CH3)2 -H2C-0-H2C-H2C- ÓH (Betaxolol) -00, c=C, H H -o-CH2-CH-CH2-NH2-C(CH3)3 (Timolol) OHarrow_forward
- Name the nitrogenous bases that are bondedarrow_forwardThe following hexoses are....arrow_forwardClassify each of the following molecular formulas as an acyclic alkane or a cycloalkane. Part 1 of 4 C2H4: acyclic alkane ☑ Part 2 of 4 C23 H48 (Choose one) Part 3 of 4 C4H10 (Choose one) Part 4 of 4 C2652 (Choose one) Х ☑ ك ☑ ⑤arrow_forward
- Consider the structure shown below. он 3 5 CH; O CH, O H CH; O 1 H-N-CH,-C-N-CH;-C-N-CH-C-N-CH-C-N-CH-C-ơ 2 H. H H Fill in the blank with an integer (1, 2, 3, 4, 5..) as shown in the diagram or to represent a specified number. A hydrophilic side chain is indicated by the numberarrow_forwardНО b) HO 1) Name each listed compound (a, b, c, d) below, 2) Arrange them in a right reaction order for corresponded conjugation, and 3) List all involved enzyme(s) in each step. OH НО- OH COOH o-t=o OH Н н OH HN H ОН H H OH HN Н OH c) НО d) HO HO HO OH COOH OH O₂PO OH XRarrow_forwardClassify the glycosidic bonds in each of the following disaccharides.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON