
Physical Chemistry
2nd Edition
ISBN: 9781133958437
Author: Ball, David W. (david Warren), BAER, Tomas
Publisher: Wadsworth Cengage Learning,
expand_more
expand_more
format_list_bulleted
Question
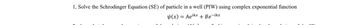
Transcribed Image Text:1, Solve the Schrodinger Equation (SE) of particle in a well (PIW) using complex exponential function
√(x) = Aeikx + Be-ikx
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- For a particle in a state having the wavefunction =2asinxa in the range x=0toa, what is the probability that the particle exists in the following intervals? a x=0to0.02ab x=0.24ato0.26a c x=0.49ato0.51ad x=0.74ato0.76a e x=0.98ato1.00a Plot the probabilities versus x. What does your plot illustrate about the probability?arrow_forwardIndicate which of these expressions yield an eigenvalue equation, and if so indicate the eigenvalue. a ddxcos4xb d2dx2cos4x c px(sin2x3)d x(2asin2xa) e 3(4lnx2), where 3=3f ddsincos g d2d2sincosh ddtanarrow_forwardIs the uncertainty principle consistent with our description of the wavefunctions of the 1D particle-in-a-box? Hint: Remember that position is not an eigenvalue operator for the particle-in-a-box wavefunctions.arrow_forward
- Prove that Ψ is normalized in a one-particle, one-dimensional system.arrow_forwardConsider the operators d/dx and d²/dx². Is y (x) Aeikx an +Be-ikx eigenfunction of these operators? If so, what are the eigenvalues? A, B, and k are real numbers. =arrow_forward4. Given these operators A=d/dx and B=x², can you measure the expectation values of the corresponding observables to infinite precision simultaneously?arrow_forward
- (a) Consider a wavefunction of the form: =1+x-1arrow_forwardIs the function w(x) = sinx an eigenfunction for the linear momentum operator, 6.? If yes, what is the eigenvalue? O Yes, the eigenvalue is -1 Yes, the eigenvalue is sinx O Yes, the eigenvalue is – in O Noarrow_forwardWhich of the following functions are eigenfunctions of the momentum operator? a)eikx, b)eax2, c)x, d)x2, e)ax+b, d)sin(x+3a)Give the corresponding eigenvalue if appropriate.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,
Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning, Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning

Physical Chemistry
Chemistry
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Wadsworth Cengage Learning,

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning