
Organic Chemistry
8th Edition
ISBN: 9781305580350
Author: William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
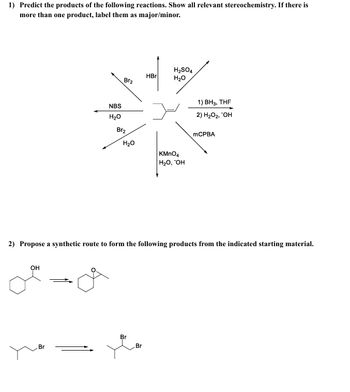
Transcribed Image Text:1) Predict the products of the following reactions. Show all relevant stereochemistry. If there is
more than one product, label them as major/minor.
Br2
HBr
H2SO4
H₂O
NBS
H₂O
Br2
H₂O
KMnO4
H₂O, OH
1) BH3, THF
2) H₂O₂, "OH
MCPBA
2) Propose a synthetic route to form the following products from the indicated starting material.
OH
Br
Br
Br
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- Provide the structure of the missing products or reactants. Show the relevant stereochemistry if necessary. H3C Br H₂O _SH [4© 1 product 2 products (2 isomers) ✓ OH + ?<arrow_forwardPredict the MAJOR product(s) of each reaction or sequence of reactions. Show stereochemistry where applicable and draw out ALL stereoisomers that are formed as MAJOR products. For those with more than one reaction, show ONLY the final product(s) after all steps listed are performed (intermediate products will not be graded). Assume all reagents are in excess. 1) PBr3 2) N3 OH 1) HBr 2) CH;00arrow_forwardBicyclo-2,5-heptadiene can be prepared in two steps from cyclopentadiene and vinyl chloride. Provide a mechanism for each step.arrow_forward
- CS41 10.) Chapter 7: E2 SNI + EI SN1+ E1 Mechanisms. Using correct mechanistic arrows, provide the step-by-step mechanism that demonstrates how the following reactions proceed to afford both substitution and elimination products via SN1 and E1. You do not need to show stereochemistry while providing the mechanism, but show correct stereochemistry in the final product(s). If there is more than one product feel free to write +E or +D as appropriate (or just write your products). C341 Br CH3NH2 CH3CH2OH CH₂OH Br CH3CH₂OH A Page 5 of Chapter 7: E2 SNI + E1arrow_forwardBr Brz CH3 CH3 H3C CH2CI2 H3C Br Electrophilic addition of bromine, Br2; to alkenes yields a 1,2-dibromoalkane. The reaction proceeds through a cyclic intermediate known as a bromonium ion. The reaction occurs in an anhydrous solvent such as CH,Cl). In the second step of the reaction, bromide is the nucleophile and attacks at one of the carbons of the bromonium ion to yield the product. Due to steric clashes, the bromide ion always attacks the carbon from the opposite face of the bromonium ion so that a product with anti stereochemistry is formed. Draw curved arrows to show the movement of electrons in this step of the mechanism. Arrow-pushing Instructions Br: :Br: .CH3 H3C H3C CH3 Br:arrow_forwardPredict the major organic product for the following reaction. You do not need to account for stereochemistry, but be sure your answer accounts for regiochemistry where appropriate. H,C CH CH, Product?arrow_forward
- Determine the predominant type of reaction: SN1, SN2, E1 and E2. Draw the major products only. Show stereochemistry whenever applicable.arrow_forwardEach of these reaction produces ONE MAJOR PRODUCT. In each case, draw this product in the box provided, including appropriate stereochemistry when applicable. For multi-step reactions, just give the FINAL product, no intermediates.arrow_forwardPlease show the major products with appropriate stereochemistry where needed.arrow_forward
- 1) Predict the products of the following reactions. a) PhCH₂CH₂CH₂Br + excess NH₂ b) 1-bromopentane c) NO₂ NaN) LIAIH Sn, HCIarrow_forwardA student adds NBS to a solution of 1-methylcyclohexene and irradiates the mixture with a sunlamp until all the NBS has reacted. After a careful distillation, the product mixture contains two major products of formula C7H11Br. Draw the products obtained from each free-radical intermediate.arrow_forwardI was able to answer correctly but I would like more clarrifiation on why this is the correct answer.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning