
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:U
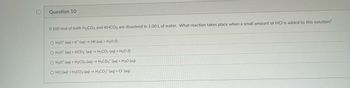
Question 10
0.100 mol of both H₂CO3 and KHCO3 are dissolved in 1.00 L of water. What reaction takes place when a small amount of HCI is added to this solution?
O H30* (aq) + K* (aq) → HK (aq) + H₂O (1)
O H30* (aq) + HCO3 (aq) → H₂CO3(aq) + H₂O (1)
O H30* (aq) + H₂CO3 (aq) → H3CO3+ (aq) + H₂O (aq)
O HCI (aq) + H₂CO3 (aq) → H3CO3+ (aq) + Cl (aq)
Expert Solution
arrow_forward
Step 1
In buffer solution, one is acidic part and another is basic part.
So when an acid is added to buffer , it will react with basic part to form acid and water.
When a base is added , it will react with acid part to form basic part.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- If you begin with 28.4 mL of 0.287 M HCl(aq) solution and mix it with 17.3 mL of 0.181 M NaOH(aq), what is the final pH when they are mixed together?HCl(aq)+ NaOH(aq)→ H2O(l) + NaCl(aq)arrow_forwardWrite a balanced net-ionic equation for the neutralization reaction between CH3COOH (aq) and Ba(OH)2 (aq). 2 CH3COOH (aq) + Ba(OH)2 (aq) --> 2 CH3COO- (aq) + 2 H₂O (1) + Ba²+ (aq) 2 CH3COOH (aq) + 2 OH" (aq) --> 2 CH3COO(aq) + 2 H₂O (1) CH3COOH (aq) + OH" (aq) --> CH3COO (aq) + H₂0 (1) OH(aq) + OH- (aq) --> H₂O (1) 2 H (aq) + 2 OH- (aq) --> 2 H₂O (1)arrow_forwardSodium hydrogen carbonate NaHCO3 , also known as sodium bicarbonate or "baking soda", can be used to relieve acid indigestion. Acid indigestion is the burning sensation you get in your stomach when it contains too much hydrochloric acid HCl , which the stomach secretes to help digest food. Drinking a glass of water containing dissolved NaHCO3 neutralizes excess HCl through this reaction: HCl (aq) + NaHCO3 (aq) → NaCl (aq) + H2O (l) + CO2 (g)The CO2 gas produced is what makes you burp after drinking the solution. Suppose the fluid in the stomach of a woman suffering from indigestion can be considered to be 150.mL of a 0.053 M HCl solution. What mass of NaHCO3 would she need to ingest to neutralize this much HCl ? Be sure your answer has the correct number of significant digits.arrow_forward
- Classify the following unbalanced chemical reactions as neutralization or precipitation reactions.arrow_forwardSodium hydrogen carbonate NaHCO3 , also known as sodium bicarbonate or "baking soda", can be used to relieve acid indigestion. Acid indigestion is the burning sensation you get in your stomach when it contains too much hydrochloric acid HCl , which the stomach secretes to help digest food. Drinking a glass of water containing dissolved NaHCO3 neutralizes excess HCl through this reaction: HCl (aq) + NaHCO3 (aq) → NaCl (aq) + H2O (l) + CO2 (g)The CO2 gas produced is what makes you burp after drinking the solution. Suppose the fluid in the stomach of a woman suffering from indigestion can be considered to be 150.mL of a 0.023 M HCl solution. What mass of NaHCO3 would she need to ingest to neutralize this much HCl ? Be sure your answer has the correct number of significant digits.arrow_forwardI understand that the balanced equation is: CaCl2(aq)+K2CO3(aq)=CaCO3+2KCL However, I don't know how to tell which is a solid (s) and which is (aq) in the final balanced part. Can someone explain this part please? Thanksarrow_forward
- Sodium hydrogen carbonate (NaHCO3), also known as sodium bicarbonate or "baking soda", can be used to relieve acid indigestion. Acid indigestion is the burning sensation you get in your stomach when it contains too much hydrochloric acid (HCI), which the stomach secretes to help digest food. Drinking a glass of water containing dissolved NaHCO3 neutralizes excess HCl through this reaction: HCl(aq) + NaHCO3(aq) NaCl(aq) + H2O(l) + CO₂(9) The CO2 gas produced is what makes you burp after drinking the solution. Suppose the fluid in the stomach of a woman suffering from indigestion can be considered to be 250. mL of a 0.076 M HCl solution. What mass of NaHCO3 would she need to ingest to neutralize this much HCl ? Round your answer to 2 significant digits. x10 Garrow_forwardSodium hydrogen carbonate NaHCO3, also known as sodium bicarbonate or "baking soda", can be used to relieve acid indigestion. Acid indigestion is the burning sensation you get in your stomach when it contains too much hydrochloric acid HCl, which the stomach secretes to help digest food. Drinking a glass of water containing dissolved NaHCO3 neutralizes excess HCl through this reaction: HCl(aq)+NaHCO3(aq)→NaCl(aq)+H2O(l)+CO2(g) The CO2 gas produced is what makes you burp after drinking the solution. Suppose the fluid in the stomach of a woman suffering from indigestion can be considered to be 200.mL of a 0.089M HCl solution. What mass of NaHCO3 would she need to ingest to neutralize this much HCl? Be sure your answer has the correct number of significant digits.arrow_forwardWhat is the net ionic reaction for the titration of hydrobromic acid with sodium hydroxide? Group of answer choices OH−(aq) + HBr(aq) → Br−(aq) + H2O(l) OH−(aq) + H+(aq) → H2O(l) NaOH(aq) + HBr(aq) → NaBr(aq) + H2O(l) NaOH(aq) + H+(aq) → H2O(l) + Na+(aq)arrow_forward
- A student titrates a 35.00 mL sample of 0.0100 M sodium hypochlorite (NaClO), a salt containing the conjugate base of hypochlorous acid (HClO, Ka = 3.0 x 10-8), with 0.0125 M HCl. NaClO(aq)+ HCl(aq) → NaCl(aq)+ HClO(aq) Determine the pH after 12.00 mL of HCl has been added.arrow_forwardThe following chemical reaction takes place in aqueous solution: 2AgNO3 (aq) + NH42S (aq) → Ag2S (s) + 2NH4NO3 (aq)Write the net ionic equation for this reaction.arrow_forwardMacmillar For the chemical reaction HCN(aq) + KOH(aq) → H,O(l) + KCN(aq) write the net ionic equation, including the phases. net ionic equation:arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY