
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Which resonance structure in each of the following pairs is more stable? Why?
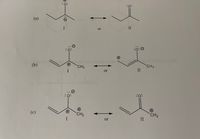
Transcribed Image Text:The image presents a series of resonance structures for different anionic and cationic species. Each subsection (a, b, and c) illustrates two resonance forms (I and II), which are depicted with arrows showing their reversible nature. Here is a detailed explanation for each section:
### (a) Resonance Structures
- **Structure I**: Depicts a molecule with a negatively charged oxygen atom (indicated by a "−" sign) attached to a two-carbon chain. The charge on the oxygen shows it has three lone pairs of electrons.
- **Structure II**: The negative charge is on the oxygen, which is part of a carbon-oxygen double bond. This structure also includes a two-carbon chain joined to the carbon atom of the carbonyl group.
Both structures are interconvertible, as shown by the double-headed resonance arrow.
### (b) Resonance Structures
- **Structure I**: Features a molecule with a positively charged carbon (cationic center) next to a negatively charged oxygen. The carbon is bonded to a methyl group (CH₃) and forms part of a three-carbon chain.
- **Structure II**: Displays the same arrangement where the positive charge is again on a carbon atom linked to the methyl group. The oxygen carries a negative charge and is double-bonded to a carbon atom.
These structures are in resonance equilibrium, as indicated by the double-headed arrow.
### (c) Resonance Structures
- **Structure I**: Shows a molecule with a negative charge on oxygen, represented by three lone pairs around the oxygen atom. There is a double bond with an aliphatic chain that ends with a CH₂⁺ group.
- **Structure II**: Illustrates a very similar arrangement but with the carbon-oxygen double bond within the molecule, maintaining the positive charge on a carbon-atom (CH₂⁺) at one end.
Similar to the previous examples, these two structures are indicated as resonance forms with a double-headed arrow.
Each set of structures highlights the delocalization of electrons in their respective systems. The charges and lone pairs fluctuate between different atoms, leading to more stable resonance hybrids overall.
Expert Solution
arrow_forward
Step 1
Resonance structure are the structure of a molecule which are obtained by conjugation of π electrons .
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- 3) Draw two more resonance structures of the following compound. Order the structures by increasing stability.arrow_forwardConsider a chemical species (either a molecule or an ion) in which a carbon atom forms three single bonds to three hydrogen atoms and in which the carbon atom possesses an unshared electron pair. (a) What formal charge would the carbon atom have? Note: enter sign followed by number to answer this question (e.g. "+1"). Formal charge = (b) What total charge would the species have? Note: enter sign followed by number to answer this question (e.g. "+1"). Total charge = (c) What shape would you expect this species have? Shape= (d) What would you expect the hybridization of the carbon atom to be? Carbon hybridization = sp O sp³d² O sp³d O sp³ sp²arrow_forwardDraw the reasonable resonance structures #2N 애arrow_forward
- Which of these best descibe formal charge? Select all that apply. The difference between the number of electrons around an atom in the free state and the number of electrons assigned to the atom in the Lewis structure an atom in a chemical compound. O The formal charge of each atom is calculated by subtracting the number of valence electrons in the neutral atomfrom the number of electrons assigned to the atom. O Can be used to help determine the most reasonable distribution of electrons in a molecule or ion. O The charge that an atom in a molecule or ion would have if all atoms had the same electronegativity.arrow_forwarddraw best resonance structuresarrow_forwardNhy is resonance structure A and resonance structure B not the same structure? If you rotate the molecule like a spicket handle (l. e. clockwise with the C at the center and the R group remaining in place as you look down the C - R bond) wouldn't you get the same thing? Why are these not equivalent structures? Resonance structure A t Resonance structure B Actual structurearrow_forward
- rank the following resonance forms in order of increasing contribution to the overall structure of the molecule N A N B Carrow_forwardDraw all resonance structuresarrow_forwardThe average bond dissociation energy of a carbon-carbon bond is 410 kJ/mol. What wavelength in nanometers of ultraviolet radiation has an energy of 410 kJ/mol?arrow_forward
- Draw 3 resonance structures of CNO- (connected in that order) and rank them in terms of stability.arrow_forwardWhen part of a molecule, what is the typical number of bonds and nonbonding pairs of electrons for each of these atoms? bonds to carbon: nonbonding electron pairs on carbon: bonds to oxygen: nonbonding electron pairs on oxygen: bonds to sulfur: nonbonding electron pairs on sulfur: bonds to halogens (F, Cl, Br, I): nonbonding electron pairs on halogens: bonds to nitrogen: nonbonding electron pairs on nitrogen: bonds to phosphorus: nonbonding electron pairs on phosphorus: bonds to hydrogen: nonbonding electron pairs on hydrogen:arrow_forwardDraw all the resonance structures for periodate ion.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY