
The pH of human stomach acid ranges from 1.0 to 3.0, whereas a healthy esophagus has a pH of approximately 7.0. In gastroesophageal reflux disease (GERD), often called acid reflux, stomach acid flows backward from the stomach into the esophagus. Repeated episodes in which esophageal pH goes below 4.0, considered clinical acid reflux, can result in bleeding ulcers and damage to the esophageal lining. The data in the Figure, from a patient with GERD, show esophageal pH during a sleeping reflux event.
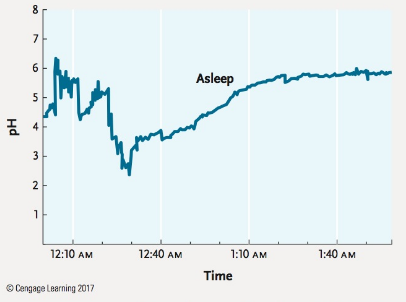
How many minutes does it take to go from the peak of the reflux event (when pH is most acidic) to when the reflux event is over?
Source: Based on T. Demeester et al. 1976. Patterns of gastroesophageal reflux in health and disease. Annals of Surgery 184:459–469.
Want to see the full answer?
Check out a sample textbook solution
Chapter 2 Solutions
Biology: The Dynamic Science (MindTap Course List)
- A patient is suspected of having low stomach acid, a condition known as hypochloridia. To determine whether the patient has this condition, her doctors take a 18.00 mL sample of her gastric juices and titrate the sample with 3.94 x 10-4 M KOH. The gastric juice sample required 6.60 mL of the KOH titrant to neutralize it. Calculate the pH of the gastric juice sample. Assume the sample contained no ingested food or drink which might otherwise interfere with the titration. pH = Enter numeric value For the patient to be suffering from hypochloridia, the pH of the gastric juices from the stomach must be greater than pH 4. Does the patient have hypochloridia? no O unable to determine O yesarrow_forwardA sample was analysed for renal and liver function tests. The sample was from a 50-year- old man who had seen his GP to report problems with tiredness. The results observed were reviewed (reference ranges are given in brackets): Sodium Potassium Urea Creatinine Alkaline phosphatase Alanine aminotransferase Albumin Bilirubin Explain these results. 200 mmol/L >10 mmol/L 6.2 mmol/L 87 μmol/L 153 IU/L 34 IU/L 40 g/L 12 μmol/L (135-145) (3.5-5.0) (3.5-6.6) (70-150) (95-320) (5-42) (35-50) (<17)arrow_forwardConsider the decapeptide angiotensin I. Treatment of angiotensin I with ACE (the angiotensin-convertingenzyme) cleaves only the amide bond with the carbonyl group derivedfrom phenylalanine to afford two products. The larger polypeptide isangiotensin II, a hormone that narrows blood vessels and increasesblood pressure. Give the amino acid sequence of angiotensin II usingthree-letter abbreviations. ACE inhibitors are drugs that lower bloodpressure by inhibiting the ACE enzymearrow_forward
- Using the Friedewald equation, compute for the LDL-chol. of the patient.Use the mmol/L, so, if you have results in mg/dL like HDL, convert it first to mmol/L. Triglyceride concentration : 0.679mmol/L Cholesterol concentration: 4.843 mmol/L HDL: 49.3 mg/dLarrow_forwardThe of the principal effects of diarrhea is the excretion (elimination through the bowels) of large quantities of sodium bicarbonate. Draw the equilibrium reaction showing the bicarbonate equilibrium. In which direction does the bicarbonate buffer system shift under these circumstances?arrow_forwardThe pH level of the human blood must be maintained at a pH within 7.35-7.45. Excess acidity or basicity in the blood, brought about by the food we take, would lead to disorder in the metabolic processes; at times, the right amount of oxygen will not be circulated to the tissues. Our natural filter, the kidneys, help preserve the normal pH level of the body. In at most five (5) sentences, discuss ways that we can do so as not to overwork our kidneys in order to maintain the pH balance of our body.arrow_forward
- Use a calculation based on the pH partition model to determine the likelihood that a weakly acidic drug of pKa= 3.0 will be absorbed passively from the stomach (pH = 2.3) or from the small intestine (pH = 5.0). The pH of the blood is 7.4 .arrow_forwardA cholesterol screening has reported Toms blood serum contains 50mg/dal HDL and 180 mg/dal LDL and a total cholesterol of 255. What is the LDL/HDL ratio? Is this value considered in the healthy range?arrow_forwardCalcium carbonate (CaCO₃(s)) is an important building material (limestone) and a quick cure for acid indigestion (TUMS). Ksp = 8.7x10⁻⁹ for CaCO₃(s). What, if any, is the effect of lowering the pH (as occurs in acid rain and acid indigestion) on the solubility of CaCO₃(s). (hint: use Le Chatelier's principle) A) there is no effect from lowering pH on CaCO₃(s) solubility B) lowering pH increases the CaCO₃(s) solubility C) additional information is required to determine the outcome D) lowering the pH lowers the solubilityarrow_forward
- Identify the nitrogen balance of a patient who eats 80 grams of protein per day,with a urinary excretion of 1.7 liters of urine having a nitrogen concentrationof 500 milligrams/ 100 mL.arrow_forwardHemodialysis is a process by which a machine is used to filter urea and other waste products from an individual’s blood when the kidneys fail. The concentration of urea in the blood is often modeled as exponential decay by the following function: −Kt c(t)=c0e V where K is the mass transfer coefficient (in mL/min), c(t) is the urea concentration in the blood at time t (in mg/mL) and V is the blood volume., and c0 is the initial concentration at time t = 0. Answer the following questions:(a) How long should a patient be put on dialysis to reduce the blood urea concentration from an initial value of 1.65 mg/mL to 0.60 mg/mL, given that K = 340 mL/min and V = 32, 941 mL? b) Derive a general formula for the dialysis time T , in terms of the initial urea concentration c0 and he target urea concentration c (T ) (c) The quantity Kt, is sometimes used as a measure of dialysis treatment adequacy. What are its V units and explain what does this quantity represent?arrow_forwardNitrate is more oxidized than urea. True or falsearrow_forward
 Biology: The Dynamic Science (MindTap Course List)BiologyISBN:9781305389892Author:Peter J. Russell, Paul E. Hertz, Beverly McMillanPublisher:Cengage Learning
Biology: The Dynamic Science (MindTap Course List)BiologyISBN:9781305389892Author:Peter J. Russell, Paul E. Hertz, Beverly McMillanPublisher:Cengage Learning Human Physiology: From Cells to Systems (MindTap ...BiologyISBN:9781285866932Author:Lauralee SherwoodPublisher:Cengage Learning
Human Physiology: From Cells to Systems (MindTap ...BiologyISBN:9781285866932Author:Lauralee SherwoodPublisher:Cengage Learning



