
Organic Chemistry (9th Edition)
9th Edition
ISBN: 9780321971371
Author: Leroy G. Wade, Jan W. Simek
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 15, Problem 15.41SP
An important variation of the Diels-Alder reaction is intramolecular, in which the diene and the dienophile are connected. This type of Diels-Alder reaction makes two new rings. Draw the compound produced in each of these examples; try to predict stereochemistry (using models will help). In some cases, Lewis acid catalysts are used; that can be ignored for this problem.
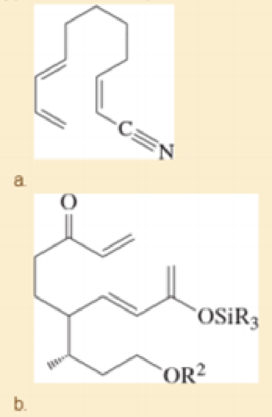
Prof. H. Miyaoka, Tokyo U. Synthesis Letters, 2011, 547. (R and R2 are different alkyl groups.)
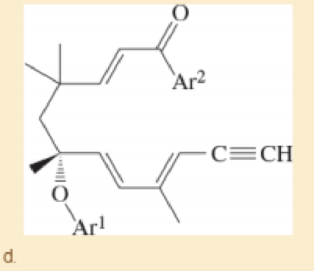
Prof. A. Kirschning, Leibnitz U. Hannover Organic Letters, 2014, 16, 568. (Ar1 and Ar2 are different aryl groups.)
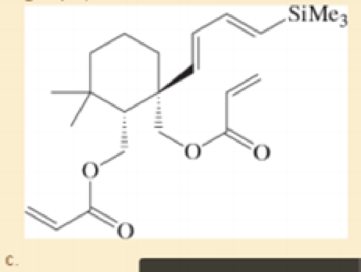
Prof. H. Zhai, Lanzhou U. Organic Letters, 2014, 16, 216.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Which of the following correctly describes the known mechanism of the Diels-Alder
reaction?
Select one:
A. A strained 4-member ring intermediate is formed which rearranges to a 6-
member ring.
B. A di-radical, 6-member ring intermediate is formed.
C. The mechanism is a one-step process with bond-making, bond breaking
changes occurring simultaneously.
D.
A zwitterion species (molecule with full +1 and -1 charges) is rapidly formed
as an intermediate.
E. A resonance-stabilized carbocation intermediate is formed in a slow step.
Refute or Defend: Once cyclopentadiene is distilled, it can be stored for a long period of time (e.g., weeks) before using it in a Diels-Alder reaction
the given copies of the starting materials to draw the major product(s) for the following Diels-Alder reaction. Use the
single bond tool to interconvert between double and single bonds, and use wedges and dashes to indicate the stereochemistry of
any newly formed chiral centers. If a pair of enantiomers is expected, be sure to draw both enantiomers. Note: you can save time
drawing by selecting a structure and using the copy/paste function.
<-{
+
Edit Drawing
01
Chapter 15 Solutions
Organic Chemistry (9th Edition)
Ch. 15.2 - Prob. 15.1PCh. 15.2 - Prob. 15.2PCh. 15.2 - Prob. 15.3PCh. 15.4 - Prob. 15.4PCh. 15.4 - Prob. 15.5PCh. 15.5 - Treatment of an alkyl halide with AgNO3 in alcohol...Ch. 15.5 - Propose a mechanism for each reaction, showing...Ch. 15.6 - When Br2 is added to buta-1,3-diene at 15 C, the...Ch. 15.7 - Prob. 15.9PCh. 15.7 - When N-bromosuccinimide is added to hex-1-ene in...
Ch. 15.7 - Prob. 15.11PCh. 15.9 - Addition of 1-bromobut-2-ene to magnesium metal in...Ch. 15.10 - Show how you might synthesize the following...Ch. 15.11 - Predict the products of the following proposed...Ch. 15.11 - Prob. 15.15PCh. 15.11A - Prob. 15.16PCh. 15.11B - Prob. 15.17PCh. 15.11B - Predict the products of the following Diels-Alder...Ch. 15.12C - Prob. 15.19PCh. 15.12C - Prob. 15.20PCh. 15.13C - Prob. 15.21PCh. 15.13D - Using the examples in Table15-2 to guide you,...Ch. 15.14 - Phenolphthalein is an acid-base indicator that is...Ch. 15 - Prob. 15.24SPCh. 15 - Prob. 15.25SPCh. 15 - Show how the reaction of an allylic halide with a...Ch. 15 - Prob. 15.27SPCh. 15 - A solution was prepared using 0.0010 g of an...Ch. 15 - Prob. 15.29SPCh. 15 - Prob. 15.30SPCh. 15 - Prob. 15.31SPCh. 15 - Prob. 15.32SPCh. 15 - Prob. 15.33SPCh. 15 - Give the structures of the products represented by...Ch. 15 - Furan and malemide undergo a Diels-Alder reaction...Ch. 15 - Prob. 15.36SPCh. 15 - Prob. 15.37SPCh. 15 - Prob. 15.38SPCh. 15 - Prob. 15.39SPCh. 15 - Determine whether each structure is likely to be...Ch. 15 - An important variation of the Diels-Alder reaction...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Imagine that you used isoprene as diene – in that case you don’t have to worry about assigning endo vs exo. Draw the "endo" and "exo" products of the Diels-Alder reaction between isoprene and maleic anhydride, and explain why the distinction is irrelevant here.arrow_forwardDraw structural formulas for the diene and dienophile that combine in a Diels-Alder reaction to form the product shown. lo Diene + Dienophile CAM • Consider E/Z stereochemistry of alkenes. • Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. • Separate multiple products using the + sign from the drop-down menu. Ⓡ Mill ChemDoodleⓇ Y -CI Sn [F CN < 46arrow_forwardDraw structural formulas for the diene and dienophile that combine in a Diels-Alder reaction to form the product shown CH3CH2O. Diene + Dienophile Co,CH3 •Consider E Z stereochemistry of alkenes. •Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. Separate multiple products using the sign from the drop-down menu. C. P. opy aste %3Darrow_forward
- The below reaction is a retro Diels-Alder reactions. Is is the reverse process of a Diels-Alder reaction with the formation of a diene and dienophile from some cyclohexene. It can be accomplished spontaneously with heat. Determine the product of the reaction. 4 Heatarrow_forwardIn Section 24.5, we learned how to use resonance OCH3 hybrids to understand the regiochemistry of Diels-Alder reactions. Frontier MO theory, on the other hand, explains the regiochemistry by taking into account the extent of orbital overlap between the frontier MOs. Consider the diene and dienophile shown here having the two different approaches indicated. In both cases, the p orbital contributions are shown for the pertinent frontier MOs. The relative contributions by the p AOs to the frontier MOs are indicated by the p AO sizes. Use this information to explain which approach is favored. CH30 LOCH3arrow_forwardFor our Diels-Alder Reaction lab, they asked to provide heat to the structures and I'm a little unsure of what the products will look like, if you can help, please?arrow_forward
- Please ex plain and Please show all arrow pushing mechaarsas. Thank you!arrow_forwardDraw structural formulas for the diene and dienophile that combine in a Diels-Alder reaction to form the product shown. • • • Diene + Dienophile H Consider E/Z stereochemistry of alkenes. Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. Separate multiple products using the + sign from the drop-down menu. ChemDoodle ?arrow_forwardIs this the correct order and conditions to produce the diels-alder product on the right? Thank youarrow_forward
- 1) In the Diels-Alder reaction there are two reaction components: a DIENE (must be a conjugated diene); and a DIENOPHILE (an ene usually with electron withdrawing substituents). a. Bicyclic diene A reacts readily with appropriate alkenes by the Diels-Alder reaction, whereas diene B is totally unreactive. Explain. b. Write the three reactions and show the product for diene A reacting with dienophiles I, II, and III. Rank I, II, and III in terms of their expected reactivity in a Diels Alder reaction. Justify your assigned ranks. A B || LOCH3 IIIarrow_forwardThe Diels-Alder Reaction THE DIELS-ALDER REACTION Pre-Laboratory Questions/Exercises Sec. No. Name 1. What problems might arise if the solvent were not anhydrous? 2. Sketch the highest occupied molecular orbital (HOMO) of butadiene, sketch the lowest unoc- cupied molecular orbital (LUMO) of ethylene, and show that these orbitals have the correct symmetry for cycloaddition. aboocne 9orollo ds To dos onRgonq blaow DoTwod worl o alilelorCIs ya 3 HOT 3. In the reaction between a 1,3-cyclopentadiene and maleic anhydride, why is the major product the more sterically hindered endo cycloadduct rather than the exo? о S O endo exo majoro minor DA O shas works h ece tarrow_forward[Review Topics] [References] Draw structural formulas for the diene and dienophile that combine in a Diels-Alder reaction to form the product shown. Diene + Dienophile CH3O CH₂ I wwwwwwwarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

Seven Name Reactions in One - Palladium Catalysed Reaction (047 - 053); Author: Rasayan Academy - Jagriti Sharma;https://www.youtube.com/watch?v=5HEKTpDFkqI;License: Standard YouTube License, CC-BY