
Concept explainers
- 12. The ideal gas law assumes that molecules bounce around and have negligible volume themselves. This is not always true. To compensate for the simplifying assumptions of the ideal gas law, the Dutch scientist Johannes van der Waals developed a “real” gas law that uses several factors to account for molecular volume and intermolecular attraction. He was awarded the Nobel Prize in 1910 for his work. The van der Waals equation is as follows:
 P, V, n, R, and T are the same quantities as found in the ideal gas law. The constant a is a correction for intermolecular forces [atm L2/mol2], and the constant b accounts for molecular volume [L/mol]. Each of these factors must be determined by experiment.
P, V, n, R, and T are the same quantities as found in the ideal gas law. The constant a is a correction for intermolecular forces [atm L2/mol2], and the constant b accounts for molecular volume [L/mol]. Each of these factors must be determined by experiment.
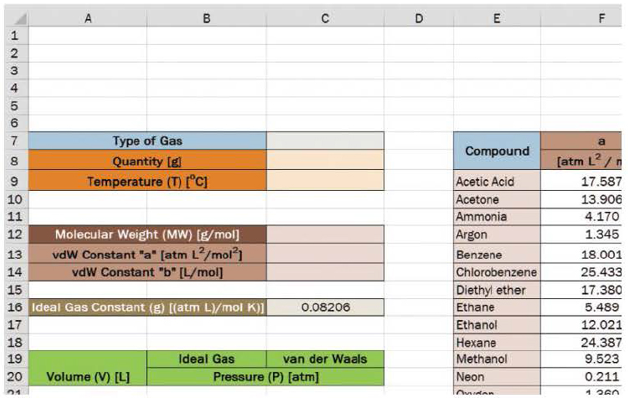
Create a worksheet using the provided template. The molecular weight, a, and b should automatically fill in after the user selects the type of gas in cell B7. The user will also set the quantity of gas and the temperature of the system.
Next, create a column of volume beginning in A21 at 0.5 liters and increasing in increments of 0.1 liters to a volume of 5 liters.
In column B, calculate the pressure (P, in atmospheres [atm]) using the ideal gas law.
In column C, calculate the pressure (P, in atmospheres [atm]) using the van der Waals equation.
Hint
Use data validation and lookup expressions using the data found in the table located in E7 to H26 in the workbook provided.
Want to see the full answer?
Check out a sample textbook solution
Chapter 10 Solutions
Thinking Like an Engineer: An Active Learning Approach (3rd Edition)
Additional Engineering Textbook Solutions
Machine Tool Practices (10th Edition)
Automotive Technology: Principles, Diagnosis, and Service (5th Edition)
Heat and Mass Transfer: Fundamentals and Applications
INTERNATIONAL EDITION---Engineering Mechanics: Statics, 14th edition (SI unit)
HEAT+MASS TRANSFER:FUND.+APPL.
Vector Mechanics for Engineers: Statics and Dynamics
- 1. For your science fair project, you decided to design a model rocket ship. The fuel burns exerting a time-varying force on the small 2.00 kg rocket model during its vertical launch. This force obeys the equation F= A + Bt2. Measurements show that at t=0, the force is 25.0 N, and at the end of the first 2.00 s, it is 45.0 N. Assume that air resistance is negligible. a. What are the forces acting on the rocket? b. Draw its free-body diagram. c. Find the constants A and B, including their SI units using this equation F= A + Bt². d. Find the net force on this rocket and its acceleration the instant after the fuel ignites. e. Find the net force on this rocket and its acceleration 3.00 s after fuel ignition. f. Suppose you were using this rocket in outer space, far from all gravity. What would its acceleration be 3.00 s after fuel ignition? g. What is the rocket's mass in outer space? What is its weight?arrow_forward4. Measured data for pressure versus volume during the expansion of gases within the cylinder of an internal combustion engine are given in the table below. Using data from the table, complete the following: a) Determine a value of n such that the data are fit by an equation of the form pV = const [1.2] b) Evaluate analytically (using an integral) the work done by the gases based on your result from part (a) [0.64 kJ] c) Using graphical or numerical integration on the data (i.e. midpoint trapezoidal rule), evaluate the work done by the gases [0.65 kJ] d) Compare the different methods for estimating the work used in parts (b) and (c). Why are they estimates? p [bar] V [cm³] 15 300 12 361 9 459 NO 6 644 4 903 2 1608arrow_forwardThe mechanism forms a structure, when the number of degrees of freedom (W) is equal toarrow_forward
- 23. The surface tension of water in contact with air is given as 0.0725 N/m. The pressure outside the droplet of 20. Determine the bulk modulus of elasticity of a fluid which is compressed in a cylinder from a volume a 0.009 m at 70 Ncn pressure to a volume of 0.0085 m' at 270 N/cm pressure. [Ans. 3.6 x 10° N/em1 21. The surface tension of water in contact with air at 20°C is given as 0.0716 N/m. The pressure inside droplet of water is to be 0.0147 N/cm greater than the outside pressure, calculate the diameter of droplet of water. 22. Find the surface tension in a soap bubble of 30 mm diameter when the inside pressure is 1.962 N/m* above [Ans. 1.94 mm) atmosphere. (Ans. 0.00735 Nm] water of diameter 0.02 mm is atmospheric 10.32 . Calculate the pressure within the droplet of cm water. [Ans. 11.77 N/em']arrow_forwardConsider an introductory thermodynamics class experiment used to demonstrate phase change phenomena. A beaker of water is heated, and its temperature measured over time to establish the temperature at which boiling occurs. The results, shown in the Figure below, are for three separate tests conducted on different days by different student groups using the same equipment and method. Why might the data from three seemingly identical tests show different results? Temperature (°C) 101 100 99 98 97 96 95 94 1 2 3 4 Time (min) 5 Boiling region 100.3 100.1 99.8 Boiling point results Test 1 (762 mm Hg) Test 2 (754 mm Hg) Test 3 (767 mm Hg) 6 7arrow_forward1. Suppose identical solid spheres are distributed through space in such a way that their centers are lie on the points of a lattice, and spheres on neighboring points just touch without overlapping. (Such an arrangement of spheres is called a close-packing arrangement.) Assuming that the spheres have unit density, show that the density of a set of close-packed spheres on each of the four structures (the "packing fraction") is: fcc: bcc: √√2/6=0.74 √√3/8=0.68 SC: π/6=0.52 √√3/16=0.34 diamond:arrow_forward
- Classical mechanicsarrow_forward1.3 A mixture of two liquids of equal volume is made, the one has a relative density of 0,8 and the other a density of 980 kg/m³. What will the weight of 2 500 litres be? 1.4 1.5 1.6 15 1.7 [21830 N] A solid block of stone with a relative density 4 is broken down and crushed to an average size of 20 mm. If the stone originally had a volume of 50 m³, how many truck loads with a volume of 2 m³, will it take to transport the crushed stone if 25 of the crushed stone has a mass of 80 kg. Has the relative density changed? How much? Why? [32 truck loads] Use table 1.4.2 to determine the kinematic viscosity of crude oil with a density of 855 kg/ m³ at temperatures of 20°C and 100°C. [9,357 x 10-6 m²/s; 3,275 x 10-6 m²/s] Air is used in a sensitive gyroscope to keep the metal bearing surfaces apart. Will the friction losses in the bearing be higher, lower or the same if the temperature of the bearing increases? A widely used S.I. unit for kinematic viscosity is centi- stoke cSt. One stoke is…arrow_forward4. One of the most common commercial brasses is the 70Cu-30Zn brass, often referred to as common or yellow brass. Locate and label its composition on the phase diagram at 200°C. a. What distinguishes 70/30 brass from the 60/40 brass you examined? b. What is the melting point of 70/30 brass? What is the melting point of 60/40 brass?arrow_forward
- The graph below shows the pressure and volume for a sample of dry air carried out at two different constant temperatures. Volume (x 10-³ m ³) 2.5 N 1.5 1 0.5 0 50 M 100 150 Pressure (k Pa) 200 250 -T= 300 K -T200 K An isothermal change has to be carried out very slowly. Explain why this is the case. Use data from the graph and the ideal gas equation to calculate the number of moles of gas in the sample.arrow_forwardMost collisions are inelastic, meaning the kinetic energy is not conserved in the system. (a) What are the three most common ways for energy to leave a system during a collision? To the best of my knowledge, the use of the word "perfectly" in a perfectly elastic collision is not necessary. Any elastic collision is a perfectly elastic collision. Both concepts mean the kinetic energy is conserved. The use of the word "perfectly" in perfectly inelastic collisions is just telling you that the two objects end up sticking to each other (have the same final velocity). (b) Combine the equations for the 1-D conservation of linear momentum and the conservation of kinetic energy to create a third equation. Any two of these equations now contain all the information, so the three equations are said to be linearly dependent in math-speak. This means we only need to use any two equations for our solutions, naturally we will chose the simplest two. (c) Use two of these equations along with…arrow_forwardThe gravitational constant g is 9.807 m/s2 at sea level, but it decreases as you go up in elevation. A useful equation for this decrease in g is g = a – bz, where z is the elevation above sea level, a = 9.807 m/s2, and b = 3.32 × 10–6 1/s2. An astronaut “weighs” 80.0 kg at sea level. [Technically this means that his/her mass is 80.0 kg.] Calculate this person’s weight in N while floating around in the International Space Station (z = 354 km). If the Space Station were to suddenly stop in its orbit, what gravitational acceleration would the astronaut feel immediately after the satellite stopped moving? In light of your answer, explain why astronauts on the Space Station feel “weightless.”arrow_forward
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





