Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter18: Functional Derivatives Of Carboxylic Acids
Section18.8: Interconversion Of Functional Derivatives
Problem AQ
Related questions
Question
100%
please help: see the pictures.
for this experiment please draw* the particular stage of the mechanism showing the purpose of adding the 6M HCl to the reaction solution. "I know the addition of 6M HCl to the reaction solution serves the purpose of converting the potassium benzilate salt formed during the reaction into the free acid form of benzilic acid". but I need help to draw the particular stage of the mechanism showing this reaction. Thanks in adavnce
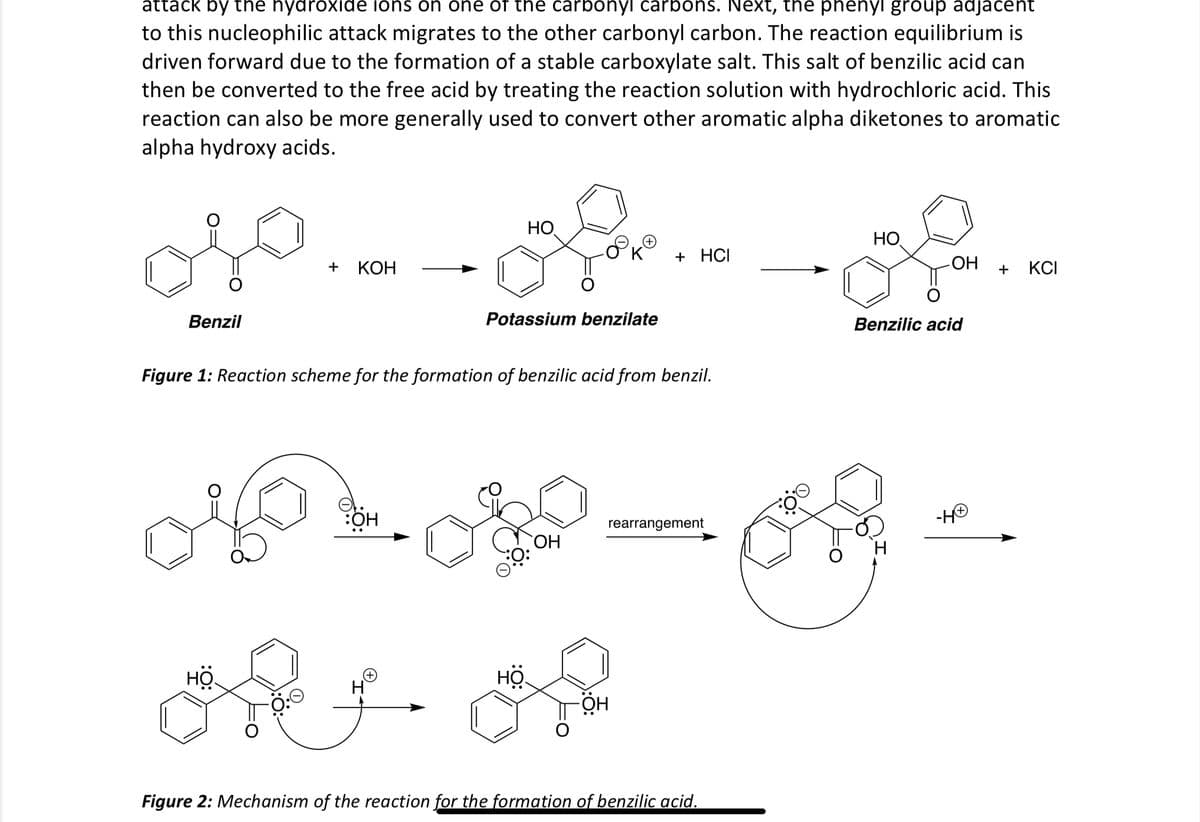
Transcribed Image Text:attack by the hydroxide ions on one of the carbonyl carbons. Next, the phenyl group adjacent
to this nucleophilic attack migrates to the other carbonyl carbon. The reaction equilibrium is
driven forward due to the formation of a stable carboxylate salt. This salt of benzilic acid can
then be converted to the free acid by treating the reaction solution with hydrochloric acid. This
reaction can also be more generally used to convert other aromatic alpha diketones to aromatic
alpha hydroxy acids.
Benzil
-Lo
HO
+ KOH
Potassium benzilate
HO
K
+ HCI
-OH + KCI
Figure 1: Reaction scheme for the formation of benzilic acid from benzil.
HO
Benzilic acid
:OH
rearrangement
OH
-H+
:ㅎ:
OH
Figure 2: Mechanism of the reaction for the formation of benzilic acid.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning