
Concept explainers
Interpretation:
The number of chemically distinct protons in the given molecule should be predicted.
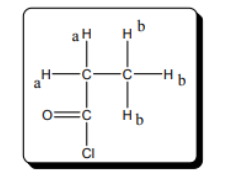
Concept Introduction:
NMR stands for nuclear magnetic resonance. A given compound contains different types of H-signals due to the presence of different magnetic environment within a molecule.
The number of signals obtained by the proton can be determined by spitting rule (n + 1), where n represents the number of the adjacent protons.
Answer to Problem 1CTQ
The number of chemically distinct proton in given molecule is 2.
Explanation of Solution
The protons present in the same environment are known as chemically equivalent protons. The number of chemically equivalent protons present in a molecule tells the number of peak obtained in the spectra for given molecule. The NMR spectra of the given molecule is represented as follows:
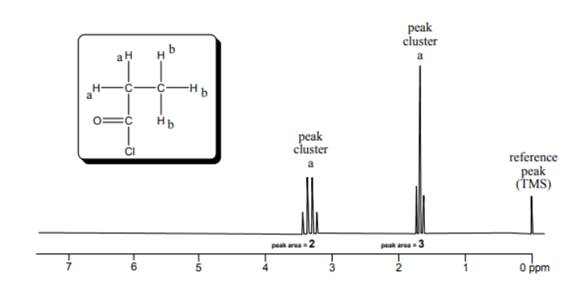
The triplet in the spectra represents that it has two neighbouring protons which means the triplet is representing protons a. on the other hand, the quartet means it has three neighbouring protons thus, representing the methyl group. As the number of peaks in the spectra is 2, that means two different chemically equivalent protons are present in the given molecule.
Thus, there are two chemically distinct protons in the model .
Want to see more full solutions like this?
Chapter L4 Solutions
Custom eBook for Organic Chemistry
- (E) What suffix do all the names in Model 1 have in common with each other?arrow_forward(CQ5) Hello! Please help me answer this one. Please refer to the given picture/s below for the questions. Please read the instructions and directions very carefully. Double and triple check your answers, previous tutors got it wrong. NOTE: Type only your answers. Please do not handwritten your answers. Make sure your formulas, solutions and answers' format are all correct. ANSWER THE LEARNING TASK 1 & 2 ONLY!arrow_forward!( plz answer with proper explanation plz)arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
