
Concept explainers
Interpretation:
The mechanism for each of the given reactions are to be written.
Concept introduction:
舧 Electrophiles are electron-deficient species, which has positive or partially positive charge. Lewis acids are electrophiles, which accept electron pair.
舧 Nucleophiles are electron-rich species, which has negative or partially negative charge. Lewis bases are nucleophiles, which donate electron pair.
舧 The reaction in which there is addition of halogen (F, Cl, Br, and I) to the compound is called halogenation reaction.
舧 A type of halogenation in which
舧 Hydride shift is defined as the rearrangement of hydrogen atoms.
Answer to Problem 1P
Solution:
(a)
(b)
(c)

(d)

Explanation of Solution
a)
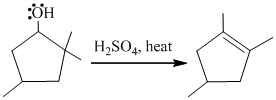
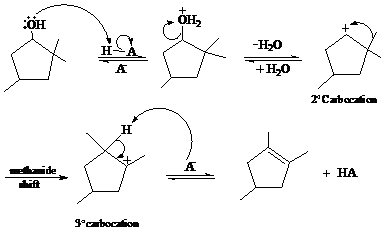
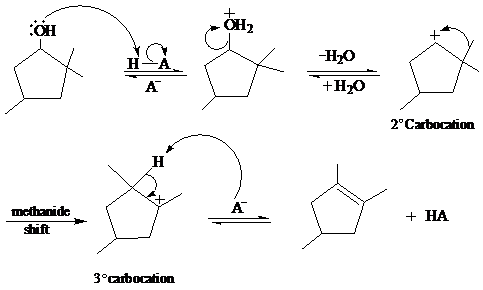
In the given reaction, cyclopentanol reacts with sulfuric acid in presence of heat to form the required product. The mechanism of this reaction takes place when the hydroxy group, which is having lone pair of electron, attacks on hydrogen atom (H) and withdraw it. After that equilibrium forms and removal of water takes place, which leads to the formation of secondary carbocation. The secondary carbocations are very unstable so methanide shift takes place and stable tertiary carbocation forms.
The mechanism of the reaction is as follows:
b)
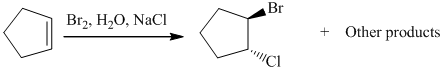
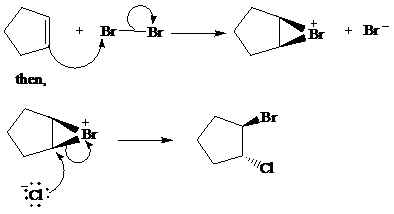
The given reaction take place when cyclopentene ring reacts with bromine in presence of light by the help of free radical halogenation. In the mechanism, the cyclopentene attacks on Br and addition of Br cation takes place at cyclopentene ring. The cyclopentene ring becomes electron deficient after the addition of Br so addition of chlorine atom takes place at cyclopentene ring and this leads to the formation of required product.
The mechanism is as follows:
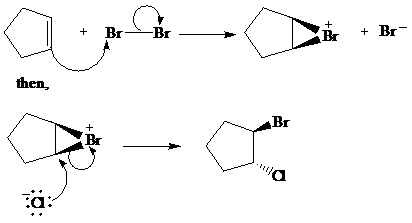
c) The other products from the reaction given in part (b).
From the above reaction, one more product is formed. When addition of chlorine atom takes place at electron deficient cyclopentene ring then addition of chlorine takes place but the product formed here is an enantiomer of the product formed above. So two products are formed, both are in equimolar amount and are enantiomer of each other.
The second product of the reaction is as follows:

d)
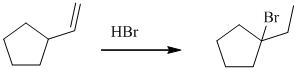
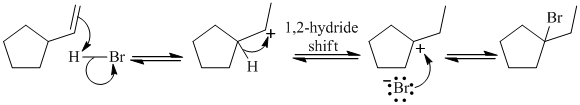
The given reaction takes place, when
The mechanism is represented below:
Want to see more full solutions like this?
Chapter FRP Solutions
Organic Chemistry, 12e Study Guide/Student Solutions Manual
- Provide the necessary reagents to accomplish the desired organic reactions. Please number the steps and note that some reactions will require more than one step. (a) (b) Me Br OMe NH Me ÓTs OMearrow_forward(c) Consider the antiallergy medication loratadine (Claritin) shown below: Loratadine (Claritin) The reaction to form the portion of the molecule in the red circle is used in the synthesis of loratadine and many other pharmaceutical agents. (i) Propose a suitable starting material and reagents to form the portion of the molecule in the red circle. (ii) Clearly outline the mechanism by which the reaction takes place. In your answer, show how the intermediate ion is stabilized.arrow_forward(b) Consider the following reaction scheme and answer the questions that follow. (i) (ii) (iv) 1 EtOH, 40 °C 2 Would compound 2 form under the given reaction conditions? Give reason(s) for your answer to question (i) above. OEt Propose a mechanism that would lead to the expected product for the above reaction scheme.arrow_forward
- Predict the major product or the necessary reagent or reactant to complete each of the following reactions. In the box before each reaction, indicate the mechanism followed by the reaction. (Free radical (FR), SN2, SN1). (b) (a) Cl₂ CH₂CH₂OH (a) 1 hv 2 (b) H Br H₂O Na+ SCH3 (c) (d) CI "CH3arrow_forward(a) Illustrate the following name reactions giving a chemical equation in each case :(i) Clemmensen reaction (ii) Cannizzaro’s reaction(b) Describe how the following conversions can be brought about :(i) Cyclohexanol to cyclohexan-1-one (ii) Ethylbenzene to benzoic acid(iii) Bromobenzene to benzoic acidarrow_forwardPredict the product(s) and provide the mechanism for each of the following reactions. (a) (b) LOCH₂CH3 HI HBr ? ?arrow_forward
- Predict the major products of the following reactions, including stereochemistry where appropriate. (a) potassium tert-butoxide + methyl iodide (b) sodium methoxide + tert-butyl iodidearrow_forwardConsider the following structural formulas. OH H;C CH3 CH3 F G H (a) Which of the compounds F-l can easily be subjected to an elimination reaction? (b) Provide the reaction mechanism for all eliminations under (a).arrow_forward(b) Alkene 6 undergoes the reaction sequence shown below to generate 7, answer ALL parts (i) to (iii) 1) Hg(OAc)2, H₂O 2) NaBH4 7 (C11H14O) 6 (i) Identify compound 7 and give a mechanism for the reaction with Hg(OAc)2 and water. [A mechanism for the reaction with NaBH4 is not required] (ii) With reference to your mechanism, explain the regioselectivity of the reaction. (iii) What would the product be if the second step was performed using deuterated sodium borohydride (NaBD4).arrow_forward
- 11:43 Q1. (a) (c) (d) (b) Two stereoisomers of but-2-ene are formed when 2-bromobutane reacts with ethanolic potassium hydroxide. (i) Explain what is meant by the term stereoisomers. Library Name and outline a mechanism for the reaction of 2-bromo-2-methylpropane with ethanolic potassium hydroxide to form the alkene 2-methylpropene, (CH3)2C=CH₂ Name of mechanism Mechanism (ii) Draw the structures and give the names of the two stereoisomers of but-2-ene. Stereoisomer 1 Name (iii) Name this type of stereoisomerism. Select Name Stereoisomer 2 When 2-bromo-2-methylpropane reacts with aqueous potassium hydroxide, 2-methylpropan-2-ol is formed as shown by the following equation. CH3 H₂C-C-CH3 + KOH Br Page 2 of 14 CH3 H3C-C-CH3 + KBr ОН State the role of the hydroxide ions in this reaction. Write an equation for the reaction that occurs when CH3CH₂CH₂CH₂Br reacts with an excess of ammonia. Name the organic product of this reaction. Equation Name of product 9,284 Photos, 1,166 Videos For You…arrow_forward(d) How would you prepare any one of the following compounds from benzene? More than one step may be involved in each case. (a) (b) OH Br m-Bromo benzoic acid Phenyl acetic acidarrow_forwardProvide the reagents required to complete the following transformations. (a) □ (b) на öd io: HỌ: 0.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





