
Concept explainers
(a)
Interpretation:
The structure for
Concept introduction:
In case of molecules containing a
If the root is a chain, numbering begins from that end of the chain which encounters the
The carbon atoms having a double or triple bond between them are always assigned C1 and C2, if the root is a ring. This must be done such that the locator numbers for the substituents are minimized. The lower of the two locator numbers for the
Answer to Problem B.26P
The structure for
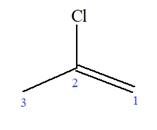
Explanation of Solution
The given molecule is
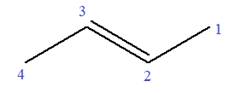
In this molecule, the root is propene. Thus, the longest carbon chain must have three carbon atoms. The suffix ‘ene’ indicates that there is a double bond in the chain. The position of the double bond in the chain is between carbon atoms C1 and C2.
The root can be shown as:
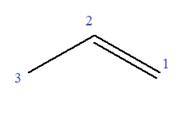
At C2 carbon atom of the root, one chlorine is attached. Thus, the structure of
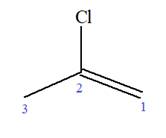
The structure of
(b)
Interpretation:
The structure for
Concept introduction:
In case of molecules containing a
If the root is a chain, numbering begins from that end of the chain which encounters the
The carbon atoms having a double or triple bond between them are always assigned C1 and C2, if the root is a ring. This must be done such that the locator numbers for the substituents are minimized. The lower of the two locator numbers for the
Answer to Problem B.26P
The structure for
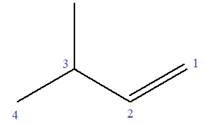
Explanation of Solution
The given molecule is
In this molecule, the root is butene. Thus, the longest carbon chain must have four carbon atoms. The suffix ‘ene’ indicates that there is a double bond in the chain. The position of the double bond in the chain is between carbon atoms C1 and C2.
The root can be shown as:
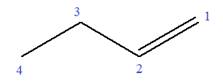
At C3 carbon atom of the root, a methyl substituent is attached.
Thus, the structure of

The structure of
(c)
Interpretation:
The structure for
Concept introduction:
In case of molecules containing a
If the root is a chain, numbering begins from that end of the chain which encounters the
The carbon atoms having a double or triple bond between them are always assigned C1 and C2, if the root is a ring. This must be done such that the locator numbers for the substituents are minimized. The lower of the two locator numbers for the
Answer to Problem B.26P
The structure for
Explanation of Solution
The given molecule is
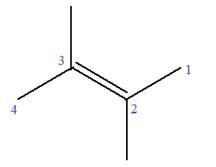
In this molecule, the root is butene. Thus, the longest carbon chain must have four carbon atoms. The suffix ‘ene’ indicates that there is a double bond in the chain. The position of the double bond in the chain is between carbon atoms C2 and C3.
The root can be shown as:
At C2 and C3 carbon atoms of the root, two methyl substituents are attached.
Thus, the structure of
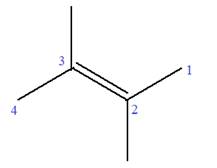
The structure of
(d)
Interpretation:
The structure for
Concept introduction:
In case of molecules containing a
If the root is a chain, numbering begins from that end of the chain which encounters the
The carbon atoms having a double or triple bond between them are always assigned C1 and C2, if the root is a ring. This must be done such that the locator numbers for the substituents, are minimized. The lower of the two locator numbers for the
Answer to Problem B.26P
The structure for
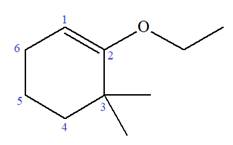
Explanation of Solution
The given molecule is
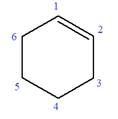
In this molecule, the root is cyclohexene. Thus, the largest carbon ring must have six carbon atoms. The suffix ‘ene’ indicates that there is a double bond in the ring. The position of the double bond in the ring is always between carbon atoms C1 and C2.
The root can be shown as:
At C2 and C3 carbon atoms of the ring, one ethoxy and two methyl substituents are attached respectively. Thus, the structure of

The structure of
(e)
Interpretation:
The structure for
Concept introduction:
In case of molecules containing a
If the root is a chain, numbering begins from that end of the chain which encounters the
The carbon atoms having a double or triple bond between them are always assigned C1 and C2, if the root is a ring. This must be done such that the locator numbers for the substituents are minimized. The lower of the two locator numbers for the
Answer to Problem B.26P
The structure for
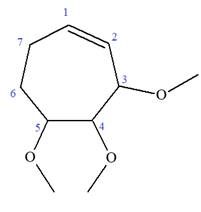
Explanation of Solution
The given molecule is
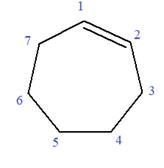
In this molecule, the root is cyclohexene. Thus, the largest carbon ring must have seven carbon atoms. The suffix ‘ene’ indicates that there is a double bond in the ring. The position of the double bond in the ring is always between carbon atoms C1 and C2.
The root can be shown as:
At C2, C3, and C4 carbon atoms of this ring, three methoxy substituents are attached.
Thus, the structure of
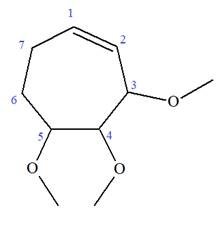
The structure of
(f)
Interpretation:
The structure for
Concept introduction:
In case of molecules containing a
If the root is a chain, numbering begins from that end of the chain which encounters the
The carbon atoms having a double or triple bond between them are always assigned C1 and C2, if the root is a ring. This must be done such that the locator numbers for the substituents are minimized. The lower of the two locator numbers for the
Answer to Problem B.26P
The structure for
Explanation of Solution
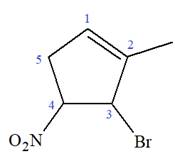
The given molecule is
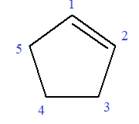
In this molecule, the root is cyclohexene. Thus, the largest carbon ring must have six carbon atoms. The suffix ‘ene’ indicates that there is a double bond in the ring. The position of the double bond in the ring is always between carbon atoms C1 and C2.
The root can be shown as:
At C2, C3, and C4 carbon atoms, bromine, methyl, and nitro group are attached.
Thus, the structure of
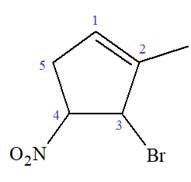
The structure of
(g)
Interpretation:
The structure for
Concept introduction:
In case of molecules containing a
If the root is a chain, numbering begins from that end of the chain which encounters the
The carbon atoms having a double or triple bond between them are always assigned C1 and C2, if the root is a ring. This must be done such that the locator numbers for the substituents are minimized. The lower of the two locator numbers for the
Answer to Problem B.26P
The structure for
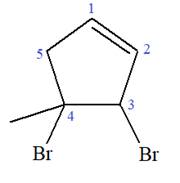
Explanation of Solution
The given molecule is
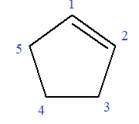
In this molecule, the root is cyclopentene. Thus, the largest carbon ring must have five carbon atoms. The suffix ‘ene’ indicates that there is a double bond in the ring. The position of the double bond in the ring is always between carbon atoms C1 and C2.
The root can be shown as:
At C3 and C4 carbon atoms of the ring, two bromine atoms and one methyl group are attached.
Thus, the structure of
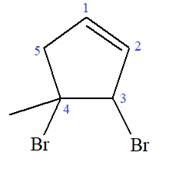
The structure of
(h)
Interpretation:
The structure for
Concept introduction:
In case of molecules containing a
If the root is a chain, numbering begins from that end of the chain which encounters the
The carbon atoms having a double or triple bond between them are always assigned C1 and C2, if the root is a ring. This must be done such that the locator numbers for the substituents are minimized. The lower of the two locator numbers for the
Answer to Problem B.26P
The structure for
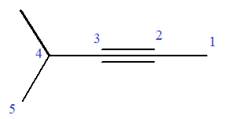
Explanation of Solution
The given molecule is
In this molecule, the root is pentyne. Thus, the longest carbon chain must have five carbon atoms. The suffix ‘yne’ indicates that there is a triple bond in the chain. The position of the triple bond in the chain is always between carbon atoms C2 and C3.
The root can be shown as:
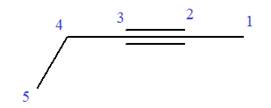
At C4 carbon atom of the root, a methyl group is attached.
Thus, the structure of
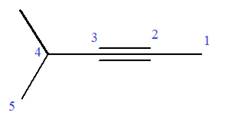
The structure of
Want to see more full solutions like this?
Chapter B Solutions
Organic Chemistry: Principles And Mechanisms
- Draw structures for the following molecules. (a) 3-bromo-2-nitropentane; (b) 2,2-dichloro-4,4,5-trinitroheptane;(c) 1,2,3,4-tetranitrobutane; (d) 6-iodo-1,2-difluorohexanearrow_forwardGiven each of the IUPAC names provided, draw the corresponding structure. (a) 1-(1,1-dimethylethyl)-2,4-diethylcyclohexane; (b) 1,4-dibutyl-2-(1-methylpropyl)cyclooctane; (c) 1,1-dicyclopropyl-3-(1,1-dimethylethyl)cycloheptanearrow_forwardDraw condensed formulas for the following compounds:(a) 3-ethyl-3-methyloctane; (b) 1-ethyl-3-propylcyclohexane (also draw a carbon-skeleton formula for this compound); (c) 3,3-diethyl-1-hexyne; (d) trans-3-methyl-3-heptene.arrow_forward
- Draw the structures of: (a) 1-ethenyl-3-nitrobenzene; (b) (1-methylpentyl)benzene; (c) 2-methyl-1,3,5-trinitrobenzene.arrow_forwardGiven each of the IUPAC names provided, draw the corresponding structure. (a) 2-cyclopropoxypentane;(b) 1,2-dimethoxy-4-propylcyclohexane; (c) 4-(1,1-dimethylethyl)-1,2-dipropoxycyclooctanearrow_forwardGiven each of the IUPAC names provided, draw the corresponding structure. (a) 1-cyclopentylhexane;(b) cyclohexylcyclohexane; (c) 1,2-dicyclopropylnonanearrow_forward
- Draw the structure of each of the following molecules (a) 2,2-dimethylcyclopentane-1-carboxylic acid;(b) (R)-3-chloropentanoic acid; (c) (2R,3S)-2,3-dinitrobutanedioic acidarrow_forwardDraw the structures for (a) 2-methyl-1,3,5-hexatriene and (b) 1,6-dimethoxyhexa-1,5-diene.arrow_forwardWrite the line-angle formula for the organic compounds (a) propene; (b) heptan-1-ol;(c) chloroacetic acid; (d) hexanoic acid.arrow_forward
- What reagents are needed to convert 1-ethylcyclohexene into (a) 1-bromo-2ethylcyclohexane; (b) 1-bromo-1-ethylcyclohexane; (c) 1,2-dibromo-1-ethylcyclohexane?arrow_forwardDraw structures for the following (a) bicyclo [3,2,0] heptane (b) spiro [4,2] heptanearrow_forwardGiven each of the IUPAC names provided, draw the corresponding structure. (a) 4-(1-methylethyl)heptane;(b) 3-(1,1-dimethylethyl)-4-(1,2-dimethylpropyl)decanearrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





