
Concept explainers
a)
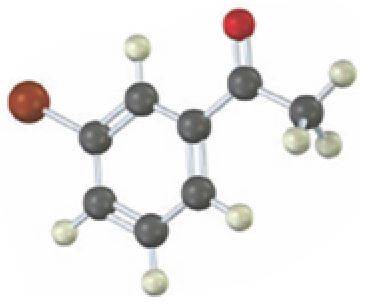
Interpretation:
Starting from which alkyne the compound shown can be prepared is to be stated.
Concept introduction:
Carbonyl compounds can be obtained by the tautomerization of enols produced when
To state:
Starting from which alkyne the compound shown can be prepared.
b)
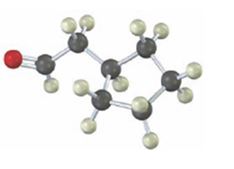
Interpretation:
Starting from which alkyne the compound shown can be prepared is to be stated.
Concept introduction:
Carbonyl compounds can be obtained by the tautomerization of enols produced when alkynes are hydrated. The hydration of terminal alkynes can yield an enol which can tautomerize to an aldehyde or ketone. If oxymercuration- demercuration hydration process is used, the OH adds to the more highly substituted carbon in the triple bond following Markovnokov regiochemistry. If hydroboration –oxidation process is used, the OH adds to the less highly substituted carbon in the triple bond following anti-Markovnokov regiochemistry.
To state:
Starting from which alkyne the compound shown can be prepared.
Want to see the full answer?
Check out a sample textbook solution
Chapter 9 Solutions
Organic Chemistry
- Are the products of the given reaction correct? Why or why not?arrow_forwardThe question below asks why the products shown are NOT the correct products. I asked this already, and the person explained why those are the correct products, as opposed to what we would think should be the correct products. That's the opposite of what the question was asking. Why are they not the correct products? A reaction mechanism for how we arrive at the correct products is requested ("using key intermediates"). In other words, why is HCl added to the terminal alkene rather than the internal alkene?arrow_forwardMy question is whether HI adds to both double bonds, and if it doesn't, why not?arrow_forward
- Strain Energy for Alkanes Interaction / Compound kJ/mol kcal/mol H: H eclipsing 4.0 1.0 H: CH3 eclipsing 5.8 1.4 CH3 CH3 eclipsing 11.0 2.6 gauche butane 3.8 0.9 cyclopropane 115 27.5 cyclobutane 110 26.3 cyclopentane 26.0 6.2 cycloheptane 26.2 6.3 cyclooctane 40.5 9.7 (Calculate your answer to the nearest 0.1 energy unit, and be sure to specify units, kJ/mol or kcal/mol. The answer is case sensitive.) H. H Previous Nextarrow_forwardA certain half-reaction has a standard reduction potential Ered +1.26 V. An engineer proposes using this half-reaction at the anode of a galvanic cell that must provide at least 1.10 V of electrical power. The cell will operate under standard conditions. Note for advanced students: assume the engineer requires this half-reaction to happen at the anode of the cell. Is there a minimum standard reduction potential that the half-reaction used at the cathode of this cell can have? If so, check the "yes" box and calculate the minimum. Round your answer to 2 decimal places. If there is no lower limit, check the "no" box.. Is there a maximum standard reduction potential that the half-reaction used at the cathode of this cell can have? If so, check the "yes" box and calculate the maximum. Round your answer to 2 decimal places. If there is no upper limit, check the "no" box. yes, there is a minimum. 1 red Πν no minimum Oyes, there is a maximum. 0 E red Dv By using the information in the ALEKS…arrow_forwardIn statistical thermodynamics, check the hcv following equality: ß Aɛ = KTarrow_forward
- Please correct answer and don't used hand raitingarrow_forwardPlease correct answer and don't used hand raitingarrow_forward(11pts total) Consider the arrows pointing at three different carbon-carbon bonds in the molecule depicted below. Bond B Bond A Bond C a. (2pts) Which bond between A-C is weakest? Which is strongest? Place answers in appropriate boxes. Weakest Bond Strongest Bond b. (4pts) Consider the relative stability of all cleavage products that form when bonds A, B, AND C are homolytically cleaved/broken. Hint: cleavage products of bonds A, B, and C are all carbon radicals. i. Which ONE cleavage product is the most stable? A condensed or bond line representation is fine. ii. Which ONE cleavage product is the least stable? A condensed or bond line representation is fine. c. (5pts) Use principles discussed in lecture, supported by relevant structures, to succinctly explain the why your part b (i) radical is more stable than your part b(ii) radical. Written explanation can be no more than one-two succinct sentence(s)!arrow_forward
- . 3°C with TH 12. (10pts total) Provide the major product for each reaction depicted below. If no reaction occurs write NR. Assume heat dissipation is carefully controlled in the fluorine reaction. 3H 24 total (30) 24 21 2h • 6H total ● 8H total 34 래 Br2 hv major product will be most Substituted 12 hv Br NR I too weak of a participate in P-1 F₂ hv Statistically most favored product will be major = most subst = thermo favored hydrogen atom abstractor to LL Farrow_forwardFive chemistry project topic that does not involve practicalarrow_forwardPlease correct answer and don't used hand raitingarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





