
a)
Interpretation:
The
Concept Introduction:
Acid dissociation constant:
Consider a weak-acid equilibrium reaction,
The acid dissociation constant
A weak acid is one that doesn’t undergo completion.
Base dissociation constant:
Consider a reaction of weak base,
The value of
Where
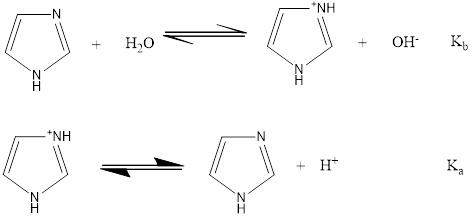
To write the chemical reaction with equilibrium constants
a)
Explanation of Solution
The chemical reaction with equilibrium constants
b)
Interpretation:
The
Concept Introduction:
Henderson-Hasselbalch equation:
Henderson- Hasselbalch equation is rearranged form of acid dissociation expression.
For acid:
Consider an acidic reaction:
The Henderson- Hasselbalch equation for an acidic reaction can be given as,
b)
Answer to Problem 9.41P
The
Explanation of Solution
Given,
Mass of Imidazole =
Mass of Imidazole hydrochloride =
Volume to be diluted =
Formula mass of Imidazole =
Formula mass of Imidazole hydrochloride =
The
The
c)
Interpretation:
The
Concept Introduction:
Henderson-Hasselbalch equation:
Henderson- Hasselbalch equation is rearranged form of acid dissociation expression.
For acid:
Consider an acidic reaction:
The Henderson- Hasselbalch equation for an acidic reaction can be given as,
c)
Answer to Problem 9.41P
The
Explanation of Solution
Given,
Mass of Imidazole =
Mass of Imidazole hydrochloride =
Volume to be diluted =
Volume and Molarity of
Formula mass of Imidazole =
Formula mass of Imidazole hydrochloride =
The
The
d)
Interpretation:
The volume in millimetres that is required to be add has to be given.
To give the required volume to be added in millimetres.
d)
Answer to Problem 9.41P
The required volume that is to be added is
Explanation of Solution
In order to obtain
Because there are
Volume of
The volume that is required of
Want to see more full solutions like this?
Chapter 9 Solutions
Quantitative Chemical Analysis
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





