
Interpretation: The continuity equation for the steady incompressible flow in the polar coordinate is to be derived using the mass conservation law.
Concept introduction:
The law of conservation of mass states that mass can only be converted from one form to another, it can neither be created nor destroyed.
The expression of the control volume for mass conservation is,
dA and dV are the area and volume of the small differential part respectively.
v = Velocity of the fluid
The sum of the net rate of mass flux out of control volume and the rate of accumulation of mass in the control volume is zero.
Answer to Problem 9.1P
The expression for the continuity equation in terms of polar coordinates is
Explanation of Solution
The mass flux through the control volume is given as,
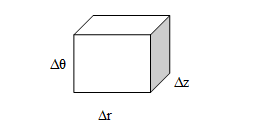
The area of the front surface =
The area of the top surface =
The area of the side surface =
Now, for equation (1), it can be written,
Also,
Substitute equation (2) and equation (3) in equation (1) and use the limit as,
The equation obtained is,
The expression for the continuity equation in terms of polar coordinates is
Want to see more full solutions like this?
Chapter 9 Solutions
Fundamentals of Momentum, Heat, and Mass Transfer
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The





