
Concept explainers
(a)
Interpretation:
The structural formula for the product of given
Concept Introduction:
Structure of the substrate plays a major role in
(a)
Answer to Problem 9.13P
The structural formula for the product of given

Explanation of Solution
In the given
Given reactant is non-chiral so product also non-chiral.

(b)
Interpretation:
The structural formula for the product of given
Concept Introduction:
Structure of the substrate plays a major role in
(b)
Answer to Problem 9.13P
The structural formula for the product of given
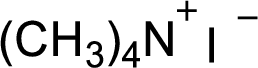
Explanation of Solution
In the given
In this reaction formed quaternary ammonium ion is stabilized by iodine ion so the product is an iodine salt of quaternary ammonium ion.
Given reactant is non-chiral so product also non-chiral.

(c)
Interpretation:
The structural formula for the product of given
Concept Introduction:
Structure of the substrate plays a major role in
(c)
Answer to Problem 9.13P
The structural formula for the product of given
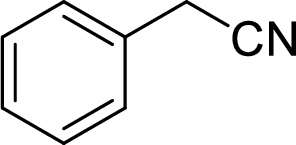
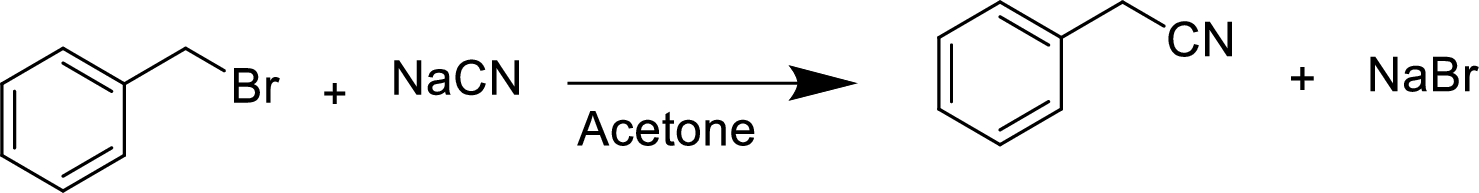
Explanation of Solution
In the given
Given reactant is non-chiral so product also non-chiral.
(d)
Interpretation:
The structural formula for the product of given
Concept Introduction:
Structure of the substrate plays a major role in
(d)
Answer to Problem 9.13P
The structural formula for the product of given
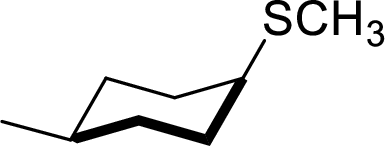
Explanation of Solution
In the given
Given reactant has an equatorial chlorine hence, the product is axial.
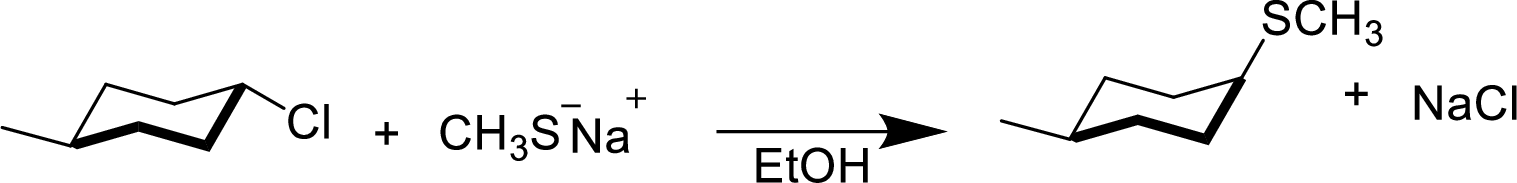
(e)
Interpretation:
The structural formula for the product of given
Concept Introduction:
Structure of the substrate plays a major role in
(e)
Answer to Problem 9.13P
The structural formula for the product of given
Explanation of Solution
In the given
Given reactant is non-chiral so product also non-chiral.

(f)
Interpretation:
The structural formula for the product of given
Concept Introduction:
Structure of the substrate plays a major role in
(f)
Answer to Problem 9.13P
The structural formula for the product of given
Explanation of Solution
In the given
In this reaction formed ammonium ion is stabilized by chorine ion so the product is a chlorine salt of ammonium ion.
Given reactant is non-chiral so product also non-chiral.
(g)
Interpretation:
The structural formula for the product of given
Concept Introduction:
Structure of the substrate plays a major role in
(g)
Answer to Problem 9.13P
The structural formula for the product of given
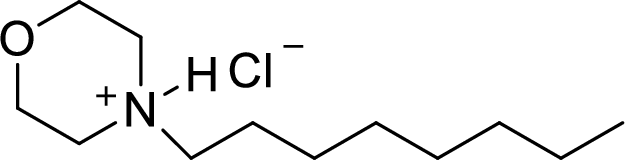
Explanation of Solution
In the given
In this reaction formed ammonium ion is stabilized by chorine ion so the product is a chlorine salt of ammonium ion.
Given reactant is non-chiral so product also non-chiral.

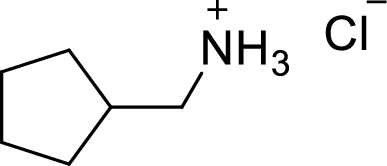
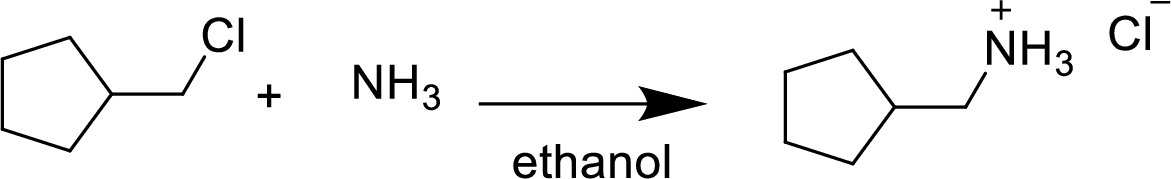
(h)
Interpretation:
The structural formula for the product of given
Concept Introduction:
Structure of the substrate plays a major role in
(h)
Answer to Problem 9.13P
The structural formula for the product of given

Explanation of Solution
In the given
Given reactant is non-chiral so product also non-chiral.

Want to see more full solutions like this?
Chapter 9 Solutions
OWL V2 with MindTap Reader and Student Solutions Manual eBook for Brown/Iverson/Anslyn/Foote's Organic Chemistry, 8th Edition
- Write an equation to show the proton transfer between each alkene or cycloalkene and HCl. Where two carbocations are possible, show each. (a) CH,CH,CH=CHCH, (b) 2-Pentene Cyclohexenearrow_forwardIn an advanced synthetic chemistry experiment, a researcher prepares a compound, ZY-7, by reacting a ketone (C5H100) with hydroxylamine (NH2OH), followed by heating in the presence of an acid catalyst. The resulting compound, ZY-7, is then treated with a solution of sodium nitrite (NaNO2) and hydrochloric acid (HCI) at low temperature. Identify the class of compound that ZY-7 most likely belongs to after this series of reactions." A) Amide B) Oxime C) Nitro compound D) Diazonium salt E) Ester Don't use chatgpt please provide valuable answerarrow_forwardKetene, H2C=C=O, is an important industrial chemical. Predict the products that would be formed when ketene reacts with **hint: Markovnikov addition occurs. (a) ethanol (b) acetic acid (c) ethylamine.arrow_forward
- An unknown hydrocarbon Q has a formula C6H12. Q Reacts with osmium tetroxide to give a diol R. When oxidized with KMnO4 in an acidic medium, Q gives two products. One product is propanoic acid and the other a ketone S. Provide reaction equations to identify the possible structures of Q, R and S.arrow_forwardIn an advanced synthetic chemistry experiment, a researcher prepares a compound, ZY-7, by reacting a ketone (C5H10O) with hydroxylamine (NH2OH), followed by heating in the presence of an acid catalyst. The resulting compound, ZY-7, is then treated with a solution of sodium nitrite (NaNO2) and hydrochloric acid (HCl) at low temperature. Identify the class of compound that ZY-7 most likely belongs to after this series of reactions." A) Amide B) Oxime C) Nitro compound D) Diazonium salt E) Ester Don't use chatgpt please provide valuable answerarrow_forwardDraw a structural formula of an alkene that undergoes acid-catalyzed hydration to give each alcohol as the major product (more than one alkene may give each alcohol as the major product). (a) 3-Hexanol (b) 1-Methylcyclobutanol (c) 2-Methyl-2-butanol (d) 2-Propanolarrow_forward
- (b) NABH, CH3 COCH,CH3 CH3CH2OH (c)arrow_forwardCompound X (structure shown below) has a molecular formula C5H1o and reacts with H2/Pt to give compound Y, C5H12. What is the name of the reaction involved to produce Compound Y? H2C H3C CH3 Hydration Hydrogenation Halogenation Addition of halohydrinarrow_forwardA compound with formula C7H12O is treated with sodium borohydride in methanol to yield 2,2-dimethylcylopentanol. Write a reaction scheme showing the structures of the reactant, the reagents, and the product. Will the product be optically active? Explain.arrow_forward
- 34) The product formed by the reaction of toluene with chlorine in the presence of sunlight is: (a) o-chlorotoluene (b) 2,4-dichlorotoluene (c) p-chlorotoluene (d) Benzylchloridearrow_forwardWrite the products of the following acid-base reactions: (a) CH3OH + H2SO4 ² ? (b) CH3OH + NANH2 2 ? (c) CH3NH3+ Cl- + NaOH ?arrow_forwardAn unknown hydrocarbon A with the formula C6H12 reacts with 1 molar equivalent of H2 over a palladium catalyst. Hydrocarbon A also reacts with OsO4 to give diol B. When oxidized with KMnO4 in acidic solution, A gives two fragments. One fragment is propanoic acid, CH3CH2CO2H, and the other fragment is ketone C. What are the structures of A, B, and C? Write all reactions, and show your reasoning.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





