
(a)
Interpretation:
The most stable carbocation structural formula of given molecular formula has to be drawn.
Concept Introduction:
The most stable carbocation structural formula:
The most stable structural arrangement of atoms in a carbocation molecule is known as most stable carbocation structural formula.
The highly alkyl substituted carbocation is more stable
Hence, the stability of carbocation is,
(a)
Answer to Problem 9.10P
The most stable carbocation structural formula of given molecular formula is,
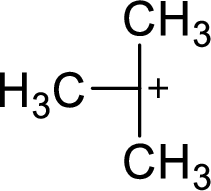
Explanation of Solution
The highly alkyl substituted carbocation is more stable. Hence, the most stable carbocation structural formula of given molecular formula is,
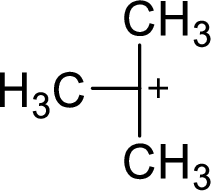
(b)
Interpretation:
The most stable carbocation structural formula of given molecular formula has to be drawn.
Concept Introduction:
The most stable carbocation structural formula:
The most stable structural arrangement of atoms in a carbocation molecule is known as most stable carbocation structural formula.
The highly alkyl substituted carbocation is more stable
Hence, the stability of carbocation is,
(b)
Answer to Problem 9.10P
The most stable carbocation structural formula of given molecular formula is,
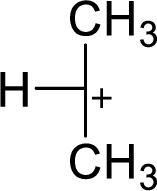
Explanation of Solution
The highly alkyl substituted carbocation is more stable. Hence, the most stable carbocation structural formula of given molecular formula is,
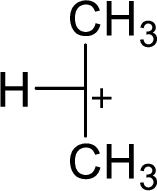
(c)
Interpretation:
The most stable carbocation structural formula of given molecular formula has to be drawn.
Concept Introduction:
The most stable carbocation structural formula:
The most stable structural arrangement of atoms in a carbocation molecule is known as most stable carbocation structural formula.
The highly alkyl substituted carbocation is more stable
Hence, the stability of carbocation is,
(c)
Answer to Problem 9.10P
The most stable carbocation structural formula of given molecular formula is,
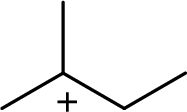
Explanation of Solution
The highly alkyl substituted carbocation is more stable. Hence, the most stable carbocation structural formula of given molecular formula is,
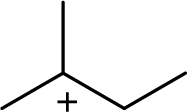
(d)
Interpretation:
The most stable carbocation structural formula of given molecular formula has to be drawn.
Concept Introduction:
The most stable carbocation structural formula:
The most stable structural arrangement of atoms in a carbocation molecule is known as most stable carbocation structural formula.
The highly alkyl substituted carbocation is more stable
Hence, the stability of carbocation is,
(d)
Answer to Problem 9.10P
The most stable carbocation structural formula of given molecular formula is,

Explanation of Solution
The highly alkyl substituted carbocation is more stable. Hence, the most stable carbocation structural formula of given molecular formula is,

Want to see more full solutions like this?
Chapter 9 Solutions
BNDL: ACP ORGANIC CHEMISTRY:CH EM 231(W/ACCESS CARD)
- Arrange the alkenes in each set in order of increasing rate of reaction with HI and explain the basis for your ranking. Draw the structural formula of the major product formed in each case. (a) and (b) H3CH₂CHC=CH₂ and (H3C) 2C=CHCH3arrow_forwardQ3. 2-Bromopentane, when treated with alcoholic KOH yields a mixture of three alkenes A, B and C. Identify A, B and C. Which is predominant? Q4 Which statement below about Sn1 reactions is incorrect? (A) SN1 reactions are stepwise and have intermediates. (B) The slow step in a SN1 reaction is formation of the carbocation intermediate. (C) SN1 reactions have first order kinetics which means only the alkyl halide is involved in the rate limiting step. (D) The products of a SN1 reaction will be a pair of enantiomers. (E) An aprotic solvent is best for Sn1 reactions as they tend to help stabilize carbocation intermediates.arrow_forwardWrite the IUPAC name for each compound, including the designation of configuration. Do not worry about italics, however syntax is important. (a) Br (b) (S)-2-bromobutane Br H3C. (c) 1-bromo-4-methylcyclohexane (d) 3-chlorocyclohexenearrow_forward
- Rank by the stability of the alkene isomers. The most stable isomer is 1, while the least stable isomer is 5. (A) (B) (C) (D) (E)arrow_forward1 Provide the IUPAC name of the following compounds, with clear indication of stereochemistry for stereocenters and alkene. (a) (b) Br (c) Me. (d) Mearrow_forwardA tautometric keto (A) – enol(B) equilibrium may be formulated as follows (A) (B) CH3 – COH ß-----------àCH2 – CHOH Given the following bond energies: C – H = 435kJ mol-1 C – C = 368 C==C = 610 C – O = 357 kJmol-1 C==O = 748 kJmol-1 O – H = 462 kJ mol-1 Calculate the enthalpy change in going from Keto form (A) to the enol form (B)arrow_forwardComplete these reactions, showing the stereochemistry of each product: CH3 (a) + Br₂ CH₂Cl₂ Complete these reactions: (b) (b) + Cl₂ CH₂Cl₂ CH₂ + Cl₂ CH₂Cl₂arrow_forward(a) Draw all stereoisomers formed by monochlorination of the cis and trans isomers of 1,2-dimethylcyclobutane drawn below. (b) How many constitutional isomers are formed in each reaction? (c) Label any pairs of enantiomers formed.arrow_forwardShow how you would convert(a) oct-3-yne to cis-oct-3-ene.(b) pent-2-yne to trans-pent-2-ene.(c) cis-cyclodecene to trans-cyclodecene.(d) but-1-yne to cis-hex-3-enearrow_forward(a) (CH3)3CBr classify the compound as a methyl, primary, secondary, or tertiary halide.arrow_forwardarrow_back_iosSEE MORE QUESTIONSarrow_forward_ios
