
a)
Interpretation:
Mention the isomer with a lower boiling point between hexane and 2, 3-dimethylbutane
Concept Introduction:
Hexane can be defined as the
Chemical formula - 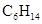
Characteristics of hexane are:
- Colorless liquid
- In pure form, it is odourless Hexane is one of the constituents of gasoline.
One of the isomer of hexane is 2, 3-dimethylbutane.
Chemical formula - 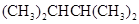
Characteristics of 2, 3-dimethylbutaneare:
- Colorless liquid
- Water insoluble
- It has a petroleum like odour
Interpretation:
Mention the isomer with a lower boiling point between pentane and 2, 2-dimethylpropane
Concept Introduction:
Pentane can be defined as the alkane which has five carbon atoms.
Chemical formula - 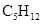
Characteristics of pentane are:
c)
Interpretation:
Mention the isomer with a lower boiling point between heptane and 2, 4-dimethylpentane
Concept Introduction:
Heptane can be defined as the alkane which is straight-chain.
Chemical formula - 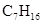
Characteristics of heptane are:
- Colorless liquid
- Water insoluble
- It has petroleum like odour
2, 4-dimethylpentane can be defined as the alkane having chemical formula 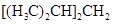
d)
Interpretation:
Mention the isomer with a lower boiling point between octane and 2, 2, 4-trimethylpentane
Concept Introduction:
Octane can be defined as the alkane and the hydrocarbon.
Chemical formula - 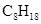
Characteristics of octane are:
- Volatile
- Very flammable
2, 2, 4-trimethylpentanecan be defined as the organic compound having chemical formula 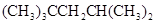
Want to see the full answer?
Check out a sample textbook solution
Chapter 9 Solutions
Chemistry For Changing Times (14th Edition)
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





