
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN: 9780618974122
Author: Andrei Straumanis
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Chapter 9, Problem 5CTQ
Interpretation Introduction
Interpretation: The given mechanism needs to be completed by adding two curved arrows.
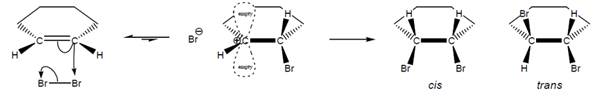
Concept introduction: When the bromine molecule reacts with the C-C double bond, the addition of 2 Br to the double bond takes place. There is the possibility of the formation of both cis and trans products. In the cis product, both bromine atoms are at a cis position to each other and in the trans product, both bromine atoms are at a trans position to each other.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
A student adds NBS to a solution of 1-methylcyclohexene and irradiates the mixture with a sunlamp until all the NBS has reacted. After a careful distillation, the product mixture contains two major products of formula C7H11Br. (a) Draw the resonance forms of the three possible allylic free radical intermediates.
2) Provide a detailed mechanism for the addition reaction shown below. State the regiochemistry
and explain why it's unusual.
Please be sure to include all structures (use line angle notation or perspective diagrams as
appropriate to illustrate the stereochemistry of the process), resonance forms, intermkediates,
transition states, curved arrows, formal charges, or lone pairs as necessary. Please don't cheat.
Br
HBr
SCH3
SCH
+
EN
for the Cl2 addition reaction with 1,2dimethylcyclohexene, draw the most reasonable curved arrow mecanism. based on the reaction products, explain why a carbocation intermediate cannot be proposed
Chapter 9 Solutions
Organic Chemistry: A Guided Inquiry
Knowledge Booster
Similar questions
- I am studying so much but I am not sure if these are correct. Can you go over it plsss? A Hoffmann product is O the result of the fastest mechanistic process. the most highly substituted alkene possible. the same as the Zaitsev's product, but the term "Hoffmann" is used for E2. the most stable alkene. What does Zaitsev's rule state? As the degree of substitution around the C=C of an alkene decreases, the stability of alkene increases. None of the statements is correct. O As the degree of substitution around the C=C of an alkene decreases, the stability of alkene decreases. As the degree of substitution around the C=C of an alkene increases, the stability of alkene decreases.arrow_forward3. Draw the mechanism of the following reaction. Include all lone pairs and formal charges. Br NaNH2 (xs), NH3 Brarrow_forwardI H Ph Br In an E2 elimination reaction, the leaving group and the hydrogen atom must be anti-coplanar for this concerted reaction to occur. ||| Identify which of the following structures has the hydrogen atom and the leaving group aligned anti-coplanar. CH3 Ph Ph H TI F H || OH H Ph H CH3 CH3 Br Ph Br A) I B) II C) IIIarrow_forward
- If the above reaction was done with chlorine instead of bromine, how many productscould form (include stereoisomers)? Show all possible products.arrow_forwardDraw the mechanism of the hydroboration reaction for 1-octene. Why are carbocation rearrangements not observed in this reaction?arrow_forwardAmong a primary, secondary, or tertiary carbocation, which is most favored for SN1 reactions? Explain whyarrow_forward
- The reaction shown proceeds via a single transition state with a trigonal bipyramidal geometry. C1 C2 Br: + H3C 0: Two curved arrows are required to indicate all of the bond-making and bond-breaking processes in this reaction. Where should one of the arrows be drawn, if CH3O is the nucleophile? from a Br LP to the O atom from C1 to the O atom from C2 to the O atom from the C-O bond to Br from an O LP to C1 from an O LP to C2arrow_forwardCan this carbocation rearrange to a more stable one?arrow_forwardDraw the major 1,2- and 1,4-addition products obtained in the reaction shown. Assume that both are derived from the most stable carbocation intermediate. + HCI • You do not have to consider stereochemistry. • You do not have to explicitly draw H atoms. • If the 1,2- and 1,4- addition products are the same due to symmetry, only draw one structure. • Draw one structure per sketcher. Add additional sketchers using the drop-down menu in the bottom right corner. ● Separate multiple products using the + sign from the drop-down menu.arrow_forward
- (b) Show the mechanism of this reaction using proper arrow push notation. You must show all intermediates, formal charges, and necessary arrows. CH3 CH3 CH3 H20 H2SO4arrow_forwardDraw the product of the below reaction, a mechanism and a Molecular Orbital ergy diagram for it. Indicate which levels are used for the reaction. MeO2C hv (light) MeO₂Carrow_forwardCurved arrows are used to illustrate the flow of electrons. Use the reaction conditions provided and follow the curved arrows to draw the product of this elementary step in an SN1 mechanism. + Include all lone pairs and charges as appropriate. Ignore stereochemistry. Ignore byproducts. Nal <arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning