
(a)
Interpretation:
The major product has to be identified.
Concept introduction:
SN1 reaction:
The reaction of alcohols with acids like hydrochloric acid or hydrobromic which yield the corresponding carbocation intermediate, this carbocation intermediate undergoes substitution reaction which yields the corresponding substitution product.
Tertiary alcohols undergo substitution very fast than the secondary alcohols because tertiary carbocation is more stable than the secondary carbocation than the primary carbocation.
Primary alcohol is less stable therefore it won’t undergoes SN1 substitution reaction.
(b)
Interpretation:
The major product has to be identified.
Concept introduction:
SN2 reaction:
The alcohols are reaction with acids like hydrochloric acid or hydrobromic which yield the corresponding substitution product. Primary alcohol undergoes SN2 substitution reaction than secondary alcohol than tertiary alcohol because SN2 reaction is simultaneous reaction.
(c)
Interpretation:
The major product has to be identified.
Concept introduction:
Dehydration reaction:
Removal of water molecule from the reaction, the alcohol is treated with strong acid like sulfuric acid.
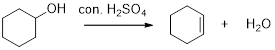
The stability of carbocation is given below,
Tertiary carbocation is more stable than the secondary and primary.
(d)
Interpretation:
The major product should be identified.
Concept introduction:
SN1 reaction:
The alcohols is reaction with acids like hydrochloric acid or hydrobromic which yield the corresponding carbocation intermediate, this carbocation intermediate undergoes substitution reaction which yields the corresponding substitution product.
Tertiary alcohols undergo substitution very fast than the secondary alcohols because tertiary carbocation is more stable than the secondary carbocation than the primary carbocation.
Primary alcohol is less stable therefore it won’t undergoes SN1 substitution reaction.
(e)
Interpretation:
The major product should be identified.
Concept introduction:
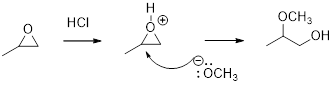
In the presence of acid catalyst, this reaction takes place through partial SN1 and partial SN2 pathway.
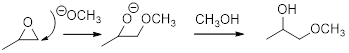
Epoxides are reactive, methoxide ion attacks the Epoxides in a less sterically hindered position which forms the alkoxide ion, and then it gets proton from alcohol which form the product.
(f)
Interpretation:
The major product should be identified.
Concept introduction:

In the presence of acid catalyst, this reaction takes place through partial SN1 and partial SN2 pathway. It is not a pure SN1 reaction because a carbocation is not formed fully and not a pure SN2 reaction because the leaving group begins to depart before the compound is attacked by the nucleophile. Epoxides are reactive; Epoxides get protonated followed by alcohol attacks to the stable carbocation and form the product.
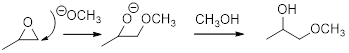
Epoxides are reactive, methoxide ion attacks the Epoxides in a less sterically hindered position which forms the alkoxide ion, and then it gets proton from alcohol which form the product. When a nucleophile attacks an unprotonated epoxide, the reaction is a pure SN2 reaction.
Note: Under acidic conditions, the nucleophile preferentially attacks the more substuituted ring carbon. Under Basic conditions, the nucleophile preferentially attacks the less substuituted ring carbon.
Want to see the full answer?
Check out a sample textbook solution
Chapter 9 Solutions
Essential Organic Chemistry (3rd Edition)
- Draw a detailed mechanism for the following reaction and identify the major product. 5. ✓ C=C_H no peroxides 2HBrarrow_forwardWhat is the major product of the following elimination reaction? H,SO, он B A В O Darrow_forwardDraw the structures for the intermediates and product C, D, and E (only) in the following synthesis. но 1) LIAIH, РСС 1) MеMgBr РСС но A → B E 2) H,Oª 2) H,Oº BH® - H,0arrow_forward
- 5) A chemist is tasked with converting tetrahydro-2H-pyran-4-ol into 4-bromotetrahydro-2H-pyran. The chemist proposed two synthetic pathways to the desired product. Choose the pathway that produces the desired product, circle one and briefly explain your choice. A complete answer will explain how the chosen pathway produces the 4-bromotetrahydro-2H-pyran AND why the other pathway was not chosen. this nathway Synthetic Pathway #1 HBr OH Synthetic Pathway #2 1) TsCl, pyridine 2) NaBrarrow_forwardThe reaction of methylpropene with HBr in ether gives one of the two products below as the major product. Br HBr Br ether Product A Product B Product would have a higher energy transition state for the formation of the intermediate leading to it. O A O B O Both products would have the same transition state.arrow_forwardMatch each of the following questions with the pool of products. WRITE THE ANSWER IN CAPITAL LETTERS. Pool of Products CAT он VAT Br BAT OH MAT Br SAT Which among the choices is the major SN2 product when the compound shown below react with NaOH? OH PAT Which among the choices is the major E2 product of compound shown below? ]]arrow_forward
- Draw the product of the following epoxide reaction, including the stereochemistry at any stereogenic centers. [1] Cl [2] H₂O Harrow_forward4arrow_forwardShow how the following starting materials are converted to the given product by a series of two pericyclic reactions. Account for the observed stereochemistry.arrow_forward
- Draw the starting structure that would lead to the major product shown under the provided conditions. Drawing H3O+ HOarrow_forwardIdentify the pericyclic reactions in the followingreaction schemes. Give the complete reaction name and indicate the course of the reaction with the aid of the arrow notation.arrow_forwardWhat is the major product from this reaction (including stereochemistry if relevant)? OH Compound O Compound P Compound Q Compound R HO P OH BH 3 THF THF; NaOH, H₂O2 H₂O Q OH ?? I R OHarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





