
Introduction:
Elements combine to form compounds. Compounds have unique characteristics. They are formed from specific combination of elements in a fixed ratio. The force that holds the atoms together in a molecule of a compound is called
Answer to Problem 1STP
Correct answer :
The correct answer is option C. CF4
Explanation of Solution
Explanation/justification for the correct answer:
Option C. CF4−Carbon has four electrons in its outermost shell and fluorine has seven electrons in its outermost shell. Onecarbon atom can share electrons with four different fluorine atoms to complete its outer energy level. It forms four covalent bonds, one with each fluorine atom.Hence one carbon atom can combine with four fluorine atoms to form a compound CF4.
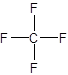
Hence this is the correct option.
Explanation for incorrect answer:
Option A. CF2−One carbon atom can share electrons with four different fluorine atoms to complete its outer energy level. It forms four covalent bonds, one with each fluorine atom. Hence one carbon atom can combine with four fluorine atoms to form a compound CF4. Carbon cannot satisfy outer energy level with only two fluorine atoms.
Hence this is not the correct option.
Option B. CF3- One carbon atom can share electrons with four different fluorine atoms to complete its outer energy level. It forms four covalent bonds, one with each fluorine atom. Hence one carbon atom can combine with four fluorine atoms to form a compound CF4. Carbon cannot satisfy outer energy level with only three fluorine atoms.
Hence this is not the correct option.
Option D. CF5- One carbon atom can share electrons with four different fluorine atoms to complete its outer energy level. It forms four covalent bonds, one with each fluorine atom. Hence one carbon atom can combine with four fluorine atoms to form a compound CF4. Carbon cannot combine with five fluorine atoms.
Hence this is not the correct option.
Chapter 9 Solutions
Biology Illinois Edition (Glencoe Science)
Additional Science Textbook Solutions
Concepts of Genetics (11th Edition)
Biology: Life on Earth with Physiology (11th Edition)
Microbiology with Diseases by Body System (4th Edition)
Biology: Life on Earth (11th Edition)
 Human Anatomy & Physiology (11th Edition)BiologyISBN:9780134580999Author:Elaine N. Marieb, Katja N. HoehnPublisher:PEARSON
Human Anatomy & Physiology (11th Edition)BiologyISBN:9780134580999Author:Elaine N. Marieb, Katja N. HoehnPublisher:PEARSON Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax
Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax Anatomy & PhysiologyBiologyISBN:9781259398629Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa StouterPublisher:Mcgraw Hill Education,
Anatomy & PhysiologyBiologyISBN:9781259398629Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa StouterPublisher:Mcgraw Hill Education, Molecular Biology of the Cell (Sixth Edition)BiologyISBN:9780815344322Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter WalterPublisher:W. W. Norton & Company
Molecular Biology of the Cell (Sixth Edition)BiologyISBN:9780815344322Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter WalterPublisher:W. W. Norton & Company Laboratory Manual For Human Anatomy & PhysiologyBiologyISBN:9781260159363Author:Martin, Terry R., Prentice-craver, CynthiaPublisher:McGraw-Hill Publishing Co.
Laboratory Manual For Human Anatomy & PhysiologyBiologyISBN:9781260159363Author:Martin, Terry R., Prentice-craver, CynthiaPublisher:McGraw-Hill Publishing Co. Inquiry Into Life (16th Edition)BiologyISBN:9781260231700Author:Sylvia S. Mader, Michael WindelspechtPublisher:McGraw Hill Education
Inquiry Into Life (16th Edition)BiologyISBN:9781260231700Author:Sylvia S. Mader, Michael WindelspechtPublisher:McGraw Hill Education





