
(a)
The mass of sugar dissolved in
(a)
Answer to Problem 1QAP
The mass of sugar dissolved in
Explanation of Solution
Given Amount of water is
Temperature is
Formula used:
Where,
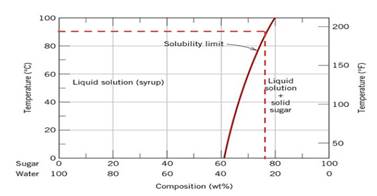
According to diagram
Calculations Put value of
Conclusion So, the mass of sugar dissolved in
(b)
Composition of saturated liquid solution at
(b)
Answer to Problem 1QAP
Composition of the saturated liquid solution is
Explanation of Solution
From the sugar-water phase diagram when the liquid saturated solution from temperature
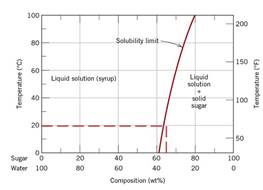
Conclusion Hence, the composition of the saturated liquid solution is
(c)
Mass of solid sugar comes out of the solution upon cooling to
(c)
Answer to Problem 1QAP
Mass of solid sugar comes out of the solution upon cooling to
Explanation of Solution
Given Amount of water is
Cooling Temperature is
Mass of sugar dissolved at
Formula used:
Solubility of sugar in water is given by:
Where,
Mass of sugar solidifies upon cooling to temperature
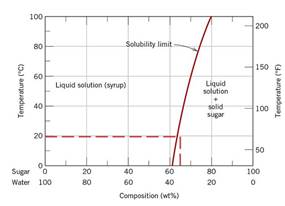
According to diagram
Calculations Put value of
Mass of sugar solidifies upon cooling to temperature
Conclusion So, the mass of sugar that solidified upon cooling to temperature
Want to see more full solutions like this?
Chapter 9 Solutions
MATERIALS SCI + ENGR: INT W/ACCESS
 MATLAB: An Introduction with ApplicationsEngineeringISBN:9781119256830Author:Amos GilatPublisher:John Wiley & Sons Inc
MATLAB: An Introduction with ApplicationsEngineeringISBN:9781119256830Author:Amos GilatPublisher:John Wiley & Sons Inc Essentials Of Materials Science And EngineeringEngineeringISBN:9781337385497Author:WRIGHT, Wendelin J.Publisher:Cengage,
Essentials Of Materials Science And EngineeringEngineeringISBN:9781337385497Author:WRIGHT, Wendelin J.Publisher:Cengage, Industrial Motor ControlEngineeringISBN:9781133691808Author:Stephen HermanPublisher:Cengage Learning
Industrial Motor ControlEngineeringISBN:9781133691808Author:Stephen HermanPublisher:Cengage Learning Basics Of Engineering EconomyEngineeringISBN:9780073376356Author:Leland Blank, Anthony TarquinPublisher:MCGRAW-HILL HIGHER EDUCATION
Basics Of Engineering EconomyEngineeringISBN:9780073376356Author:Leland Blank, Anthony TarquinPublisher:MCGRAW-HILL HIGHER EDUCATION Structural Steel Design (6th Edition)EngineeringISBN:9780134589657Author:Jack C. McCormac, Stephen F. CsernakPublisher:PEARSON
Structural Steel Design (6th Edition)EngineeringISBN:9780134589657Author:Jack C. McCormac, Stephen F. CsernakPublisher:PEARSON Fundamentals of Materials Science and Engineering...EngineeringISBN:9781119175483Author:William D. Callister Jr., David G. RethwischPublisher:WILEY
Fundamentals of Materials Science and Engineering...EngineeringISBN:9781119175483Author:William D. Callister Jr., David G. RethwischPublisher:WILEY





