
Concept explainers
Interpretation: An explanation regarding the cleavage of the given amide substrate A by chymotrypsin reveals no burst by using stopped-flow kinetic methods is to be stated.
Concept introduction: The organic compounds that contain
Proteolytic enzymes commonly known as proteases are used for the cleavage of protein. Chymotrypsin is an enzyme that is used for the cleavage of protein through hydrolysis reaction.
Answer to Problem 1P
The cleavage of the given amide substrate, A, by chymotrypsin reveals no burst because the acyl-enzyme intermediate formation occurs at a slower rate as compared to the hydrolysis for the given amide substrate.
Explanation of Solution
Given:
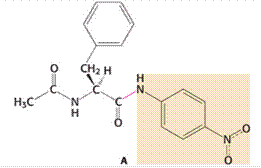
Chymotrypsin is an enzyme that is used for the cleavage of protein through hydrolysis reaction. In case of large hydrophobic amino acids, the cleavage of the peptide bond occurs particularly at the carboxyl-terminal side. In the given amide substrate A, the cleavage by chymotrypsin shows no burst because the hydrolysis for this substrate of amide is faster than the generation of the acyl-enzyme intermediate. In other words, the acyl-enzyme intermediate formation occurs at a slower rate as compared to the hydrolysis for the given amide substrate. Hence, no burst is found. In case of ester substrates, the acyl-enzyme intermediate formation occurs at a faster rate.
Want to see more full solutions like this?
Chapter 9 Solutions
SAPLINGPLUS F/BIOCHEM+ICLICKER REEF-CODE
- Question #1: Choanoflagelletes are a unicellular ancestor to animals. One observation to support this hypothesis is the appearance of adhesion molecules that are key to the development of multiceullarity. Bulk transport Gap junctions Animals. Adhesion, cell signaling Single-celled :} Insects, mammals, and other animals with bilateral symmetry (~10,000,000) Jellyfish and their relatives (10,000) } Sponges (10,000) Choanoflagellates (150) Despite their simple unicellular lifestyle they express adhesion molecules including cadherins and lectins (King et al., 2003) but don't seem to have molecules that are typically found in the extracellular matrix such as integrins or laminins (Williams et al., 2014). Design a microscopy experiment to test the assertion that choanoflagellates have (some) adhesion molecules and those molecules play a similar role in a closely related animal like sponges.arrow_forwardBased on your understanding of the structure and function of the enzyme chymotrypsin, explain why proteolytic activation by Trypsin in the intestine is required for chymotrypsin to bind its substrates with high affinity. Your answer should include a description of the amino acid residue or residues involved AND a kinetic experiment that suggested this.arrow_forwardSubstrate KM (M) N-Acetylvaline ethyl ether 8.8 X 10 -2 N-Acetyltyrosine ethyl ether 6.6 X 10-4 Which substrate has the higher apparent affinity for the enzyme? Explain. Which substrate is likely to give a higher value for Vmax?arrow_forward
- (b) The values of kinetic parameters for a variety of synthetic ester and peptide substrates ofa- chymotrypsin are compared for the reaction scheme below where ES is the Michaelis complex, ES' E+S Substrate K₁ N-Ac-Trp–OC₂H5 N-Ac-Phe-OC2H5 N-Ac-Leu-OC2H5 N-Ac-Phe-CONH2 N-Ac-Tyr-p-nitro-anilide ES K₂ 3.5 13.0 3.2 1.7 K-₁ is the acylenzyme, P₁ is the first product to be released, P2 is the second product, k2 is the acylation rate constant, k3 is the deacylation rate constant, and kcat = k2 k3/( k2 + k³). In the table below, N- Ac = N-acetyl; -CONH₂ = carboxamide; -OC₂H5 = ethyl ester; p-nitroanilide = −NH-C6H4-NO2 simulating a peptide group. ES' K2 (S-¹) K3 (S-1) 0.84 2.2 0.19 P₁ K3 0.073 H → E+ P2 Kcat (S-1) 0.82 1.9 0.18 0.070 0.038 KM (MM) 0.08 1.3 4.2 24.0 0.35 1 Kcat/ KM (mM-¹ s¯¹) 10.3 1.5 0.04 0.003 0.11 The value of kcat for N-Ac-Phe-OC2H5 is two-fold greater than that for the L-tryptophanyl analog and more than 10-fold greater than the value of kcat for the ester substrate…arrow_forwardCoenzyme-dependent enzymes can catalyze the general transformations shown below.What would be the best coenzymes to use for the two steps in the scheme, and why? Then, proposean enzyme-catalyzed method for step A to proceed without a coenzyme. Show this enzymecatalyzed mechanism for step A that does not require the coenzyme. You can abbreviate acidicamino acid residues “Enz–B–H” and basic residues “ B–Enz”.arrow_forward(i) Describe the mechanism of chymotrypsin in cleaving a peptide bond, highlighting the roles of the catalytice triad for the two phases of the catalytic reactions. Explain the significance of the oxyanion hole for the catalysis. (ii) All serine proteases contain the catalytic triad and these amino acids are positioned in the exact same conformation. Since this is true, why do trypsin and chymotrypsin have such different substrate specificity? What features of the enzyme allow for this situation?arrow_forward
- The substitution of His 64 of carbonic anhydrase II with Ala results in a sharp decrease in the activity of the enzyme in HEPES buffer (molecular weight of HEPES = 238.3 g/mol). However, increasing concentrations of imidazole (molecular weight = 68.1 g/mol) restores the reaction rate close to that of the wild-type enzyme. Propose an explanation for these results.arrow_forwardMake an electron-flow-mechanism for this synthetic scheme. This involves predicting major and by-products using electronic and structural effects. The arrow push mechanism must be shown.(from the reaction of α-ketoacids and oxaprolines to proteins that contain native serine residues ) with labelarrow_forwardHow many tritium atoms (3H) are incorporated into palmitate when fattyacid synthesis is carried out in vitro with the following labeled substrate?arrow_forward
- Using the ActiveModel for aldose reductase, describe the structure of the TIM barrel motif and the structure and location of the active site.arrow_forwardIn 1966, Ferdinand showed that a random-order ternary-complex mechanism for a two-substrate enzyme-catalysed reaction can lead to sigmoidal kinetics being observation in the absence of cooperative binding. Discuss this scenario so that it is clear why a plot of Vo versus [AXo] at constant [Bo] will be sigmoidal.arrow_forwardMultivitamin B complex are essential compounds that are used as derivatives for compounds necessary for many metabolic activities. Give 3 vitamin B compounds and explain where and how they are used along the metabolic pathway.arrow_forward
 BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
