
Interpretation:
The given carbocation is expected to undergo any carbocation rearrangement has to be predicted.
Concept Introduction:
Carbocations are only intermediates of a reaction and not starting materials of a reaction. In other words, short-lived intermediates are carbocations. The stability of the carbocation formed is decided by the group that is attached to the charged carbon atom.
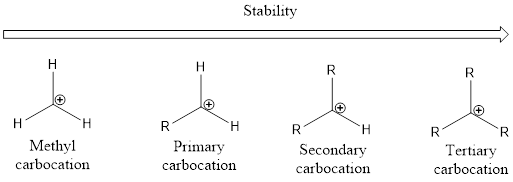
From the above, it is understood that tertiary carbocations are more stable than secondary carbocations which are more stable than primary carbocation. Unstable carbocation is the methyl carbocation. As the alkyl group increases the stability of the carbocation also increases. This is by means of phenomenon known as hyperconjugation.
Carbocations are also stabilized by resonance. The more the resonance structures that is possible for the carbocation, the more stability will be.
The carbocations possessing the positive charge at the benzylic position is more stable.
In some cases, the carbocation that is formed will try to rearrange into more stable carbocation either by shifting or resonance. In shifting, the two most common types are hydride shift and methyl shift.
Want to see the full answer?
Check out a sample textbook solution
Chapter 8 Solutions
Organic Chemistry As a Second Language: First Semester Topics
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





