
Organic Chemistry (6th Edition)
6th Edition
ISBN: 9781260119107
Author: Janice Gorzynski Smith
Publisher: McGraw Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 8.2, Problem 6P
Which
a.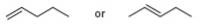 c.
c. 
b.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Rank the following alkenes from most to least stable.
A.
В.
С.
D.
B.
Problem 5.37
Compare the physical properties of the three stereoisomers of 1,3-dimethylcyclopentane.
A
B
C
three stereolsomers of 1,3-dimethylcyclopentane
a. How do the boiling points of A and B compare? What about those of A and C?
b. Characterize a solution of each of the following as optically active or optically inactive:
pure A; pure B; pure C; an equal mixture of A and B; an equal mixture of A and C.
c. A reaction forms a 1:1:1 mixture of A, B, and C. If this mixture is distilled, how many
fractions would be obtained? Which fractions would be optically active and which
would be optically inactive?
Problem 5.2
Classify each pair of compounds as constitutional isomers or stereoisomers.
a.
and
and
он
020st geabe
AA go Aon e 2o3
С.
and
d.
and
b.
Chapter 8 Solutions
Organic Chemistry (6th Edition)
Ch. 8.1 - Problem 8.1 Label the and carbons in each alkyl...Ch. 8.2 - Problem 8.2 Classify each alkene in the following...Ch. 8.2 - Prob. 3PCh. 8.2 - Prob. 4PCh. 8.2 - Problem 8.5 Label each pair of alkenes as...Ch. 8.2 - Problem 8.6 Which alkene in each pair is more...Ch. 8.2 - Problem 8.7 Several factors can affect alkene...Ch. 8.4 - Prob. 8PCh. 8.4 - Prob. 9PCh. 8.4 - Prob. 10P
Ch. 8.4 - Prob. 11PCh. 8.5 - Problem 8.12 What alkenes are formed from each...Ch. 8.6 - Prob. 13PCh. 8.6 - Problem 8.14 What alkenes are formed from each...Ch. 8.6 - Problem 8.15 How does each of the following...Ch. 8 - 8.24 Rank the alkenes shown in the ball-and-stick...Ch. 8 - Prob. 25PCh. 8 - 8.26 What is the major E2 elimination product...Ch. 8 - Prob. 27PCh. 8 - Prob. 28PCh. 8 - Prob. 29PCh. 8 - 8.30 Label each pair of alkenes as constitutional...Ch. 8 - Prob. 31PCh. 8 - Prob. 32PCh. 8 - Prob. 33PCh. 8 - For each of the following alkenes, draw the...Ch. 8 - Prob. 35PCh. 8 - Prob. 36PCh. 8 - Prob. 37PCh. 8 - What alkene is the major product formed from each...Ch. 8 - Prob. 39PCh. 8 - Prob. 41PCh. 8 - Draw the products formed when each dihalide is...Ch. 8 - Draw all of the substitution and elimination...Ch. 8 - Prob. 56PCh. 8 - 8.59 Draw a stepwise, detailed mechanism for each...Ch. 8 - Draw the major product formed when...Ch. 8 - Draw a stepwise, detailed mechanism for the...Ch. 8 - Explain why the reaction of with gives ...Ch. 8 - Draw a stepwise detailed mechanism that...Ch. 8 - Prob. 63PCh. 8 - 8.65 Explain the selectivity observed in the...Ch. 8 - Prob. 65PCh. 8 - Prob. 66PCh. 8 - 8.68 (a) Draw all products formed by treatment of...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Label each statement about the polynucleotide ATGGCG as true or false. The polynucleotide has six nucleotides. ...
General, Organic, & Biological Chemistry
Classify each example of molecular art as a pure element, a pure compound, or a mixture.
General, Organic, and Biological Chemistry - 4th edition
Practice Problem 1.22 Which of the following alkenes can exist as cis-trans isomers? Write their structures. Bu...
Organic Chemistry
The smallest building blocks inside your cell phone are about 1000 times smaller than the diameter of a human h...
Chemistry In Context
Determine [OH], [H+], and the pH of each of the following solutions. a. 1.0 M KCl b. 1.0 M KC2H3O2
Chemistry
Give the IUPAC name for each compound.
Organic Chemistry
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Problem 5.20 Label the two stereogenic centers in dB re stereoisomers. ne. each compound and draw all possible CI a. rro OH Br CI ne b. B. Problem 5.21 Compounds E and F are two isomers of 2,3-dibromopentane drawn in staggered conformations. Which compounds (A-D) in Figure 5.8 are identical to E and F? im et wihr boe A mot Br omin oldne Br F Brarrow_forwardAnswer the following questions about compounds A–D.a.How are the compounds in each pair related? Choose from constitutional isomers, stereoisomers, or identical molecules: A and B; A and C; B and D. b.Label each compound as a cis or trans isomer. c.Draw B as a hexagon with wedges and dashed wedges to show the stereochemistry of substituents. d.Draw a stereoisomer of A as a hexagon using wedges and dashed wedges to show the orientation of substituents.arrow_forwardWhich heterocycles are aromatic? a. d. b.arrow_forward
- Draw the products formed when each diene is treated with one equivalent of HCI. a. b. d.arrow_forwardProblem 5.16 Label each stereogenic center as R or S. Br b. ge, HO н он ca C. t d. OHe o lobonot b d or HOP "OH f. HO Harrow_forwardDraw the products of radical chlorination and bromination of each compound. For which compounds is a single constitutional isomer formed for both reactions? What must be true about the structure of a reactant for both reactions to form a single product? a. b. C. d. е.arrow_forward
- Draw the product of each Diels-Alder reaction and indicate the stereochemistry at all stereogenic centers. A a. b.arrow_forwardElectrophilic Addition Soubong neblA-aleid rose 9160910 31 babeen ene singo 14.43 Draw the products formed when each compound is treated with one equivalent of HBr. a. b. C.arrow_forwardWhat type of sigmatropic rearrangement is illustrated in each equation? a. b. Darrow_forward
- 10.64 Devise a synthesis of each compound from cyclohexene as the starting material. More than one step is needed. a. CO b. C. CN OH "SH + enantiomerarrow_forwardProblem 13.34 Synthesize each compound from cyclohexanol, ethanol, and any other needed reagents. a. b. C. d. e. OH XOH Br OHarrow_forwardLabel each compound as aromatic, antiaromatic, or not aromatic. Assume all completely conjugated rings are planar. Å a. b. C. d.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Nomenclature: Crash Course Chemistry #44; Author: CrashCourse;https://www.youtube.com/watch?v=U7wavimfNFE;License: Standard YouTube License, CC-BY