
Organic Chemistry (9th Edition)
9th Edition
ISBN: 9780321971371
Author: Leroy G. Wade, Jan W. Simek
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 8.15C, Problem 8.37P
Predict the major products of the following reactions.
- a. (E)-3-methyloct-3-ene + ozone, then (CH3)2 S
- b. (Z)-3- methyloct -3-ene + warm, concentrated KMnO4
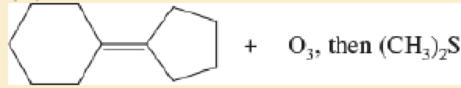
- c. 1- ethylcycloheptene + ozone, then (CH3)2 S
- d. 1ethylcycloheptene + warm, concentrated KMnO4
- e. 1- ethylcycloheptene + cold, dilute KMnO4
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Give the structure of the products that you would expect from the reaction of 1-butyne with
A group of researchers are tasked to synthesize pure (R)-butan-2-ol. Suggest the suitable pair of starting material and
reagent
O a. (R)-2-chlorobutane + H2O
O b. (S)-2-chlorobutane + NaOH
O c. (R)-2-chlorobutane + KOH
O d. (S)-2-chlorobutane + CH3OH
1. Write the structural formula of the major product(s) formed in each of the following reactions.
Show stereochemistry where appropriate. Use mechanisms to assist you in figuring out the
products when needed.
5
a.
T
NH,
G
H.
H+
b.
p-TSOH
(CH3)2NH
Benzene
с.
H.
1. (C6H5)3P
H.
Br
2. C6H5L¡
3.
Chapter 8 Solutions
Organic Chemistry (9th Edition)
Ch. 8.3A - Predict the major products of the following...Ch. 8.3A - a. When 1 mole of buta-1,3-diene reacts with 1...Ch. 8.3B - Predict the major products of the following...Ch. 8.3B - Show how you would accomplish the following...Ch. 8.4B - Propose a mechanism to show how...Ch. 8.4B - Predict the products of the following hydration...Ch. 8.6 - a. Propose a mochansm fortho following reaction....Ch. 8.6 - Prob. 8.8PCh. 8.6 - Prob. 8.9PCh. 8.7A - Prob. 8.10P
Ch. 8.7A - Prob. 8.11PCh. 8.7C - Prob. 8.12PCh. 8.7C - Prob. 8.13PCh. 8.7C - a. When (Z)-3-methylhex-3-ene undergoes...Ch. 8.7C - Prob. 8.15PCh. 8.7C - Prob. 8.16PCh. 8.8B - Prob. 8.17PCh. 8.8B - Prob. 8.18PCh. 8.9 - Prob. 8.19PCh. 8.9 - Prob. 8.20PCh. 8.9 - Prob. 8.21PCh. 8.9 - Prob. 8.22PCh. 8.10 - Prob. 8.23PCh. 8.10 - Prob. 8.24PCh. 8.10 - Prob. 8.25PCh. 8.11A - Prob. 8.26PCh. 8.11B - Prob. 8.27PCh. 8.11B - Prob. 8.28PCh. 8.12 - Prob. 8.29PCh. 8.13 - a. Propose a mechanism for the conversion of...Ch. 8.13 - Magnesium monoperoxyphthalate (MMPP) epoxidizes...Ch. 8.13 - Predict the major products of the following...Ch. 8.13 - When 1,2-epoxycyclohexane (cyclohexene oxide) is...Ch. 8.14C - Predict the major products of the following...Ch. 8.14C - Prob. 8.35PCh. 8.15B - Prob. 8.36PCh. 8.15C - Predict the major products of the following...Ch. 8.16A - Prob. 8.38PCh. 8.16A - Prob. 8.39PCh. 8.16B - Prob. 8.40PCh. 8.16B - Prob. 8.41PCh. 8.16C - Prob. 8.42PCh. 8.17B - Prob. 8.43PCh. 8.17B - Prob. 8.44PCh. 8.17B - Show how you would synthesize each compound,...Ch. 8 - Prob. 8.46SPCh. 8 - Prob. 8.47SPCh. 8 - Give the products expected when the following...Ch. 8 - Show how you would make the following compounds...Ch. 8 - Using 1,2-dimethylcyclohexene as your starting...Ch. 8 - Show how you would synthesize each compound using...Ch. 8 - Prob. 8.52SPCh. 8 - Show how you might use olefin metathesis to...Ch. 8 - Prob. 8.54SPCh. 8 - Prob. 8.55SPCh. 8 - Propose mechanisms consistent with the following...Ch. 8 - Prob. 8.57SPCh. 8 - Prob. 8.58SPCh. 8 - Draw a reaction-energy diagram for the propagation...Ch. 8 - Prob. 8.60SPCh. 8 - Prob. 8.61SPCh. 8 - Prob. 8.62SPCh. 8 - Prob. 8.63SPCh. 8 - Prob. 8.64SPCh. 8 - Prob. 8.65SPCh. 8 - Prob. 8.66SPCh. 8 - Prob. 8.67SPCh. 8 - Prob. 8.68SPCh. 8 - Prob. 8.69SPCh. 8 - Prob. 8.70SPCh. 8 - Prob. 8.71SPCh. 8 - Prob. 8.72SPCh. 8 - Prob. 8.73SPCh. 8 - Prob. 8.74SPCh. 8 - Prob. 8.75SPCh. 8 - Prob. 8.76SPCh. 8 - Prob. 8.77SPCh. 8 - Prob. 8.78SPCh. 8 - Prob. 8.79SP
Additional Science Textbook Solutions
Find more solutions based on key concepts
How could you separate a mixture of the following compounds? The reagents available to you are water, either, 1...
Organic Chemistry (8th Edition)
Separate the list P,F,V,,T,a,m,L,t, and V into intensive properties, extensive properties, and nonproperties.
Fundamentals Of Thermodynamics
Label each statement about the polynucleotide ATGGCG as true or false. The polynucleotide has six nucleotides. ...
General, Organic, and Biological Chemistry - 4th edition
Identify each of the following reproductive barriers as prezygotic or postzygotic. a. One lilac species lives o...
Campbell Essential Biology with Physiology (5th Edition)
56. Global Positioning System. Learn more about the global positioning system and its uses. Write a short repo...
The Cosmic Perspective (8th Edition)
Some people consider Pasteur or Koch to be the Father of Microbiology, rather than Leeuwenhoek. Why might they ...
Microbiology with Diseases by Body System (5th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Just B and C pleasesarrow_forwardProvide the product expected from each of the reactions below. If you believe that two products will be formed, show both (do not show more than two). If you believe that no reaction will occur, write NO REACTION. Indicate stereochemistry where appropriate.arrow_forwardHelp me with this. Thanks!arrow_forward
- Predict the product for the following reactions.arrow_forward2. Predict the product obtained from following reaction: CCH (i) HaSO4, H2O + H20 H&SO4 (ii) H CH,CH C CCH, Lindar cataly st excess Br2 CH,Cl2 HC=CCH3 (iii)arrow_forward9. What is the major product of the following reaction? Br2 . ..... . hv Br a- Br b- Br Br Br d- 10.What is the products of the following reaction? (i) O, H3C- ECH ........ (ii) Zn/H,0 a- CH;CHO + CH20 b- CH;COOH + HCOOH c- CH;CH;OH + CH;OH d- CH3CHO + HCOOHarrow_forward
- From the choices provided below, list the reagent(s) in order that will yield the following transformation. Br Reagents available a. HIO4 b. HC C Na+ c. CH3ONa+, CH, OH k. RCO, H d. NaCN e. CH3CH₂S Na+ f. H₂O+ g. H₂O h. H₂ CrO4 Your answer: i. H₂SO4, H₂O j. potassium tert-butoxide I. HN(CH, CH3 ) m. NBS, hv n. OsO4, H₂O2 NaOH O. p. pyridinium chlorochromate (PCC) List your answer as a series of letters in the order the reagents are used (with no commas separating them). No more than four steps are required for this synthesis. Use the minimum number of steps possible. Reagents may be used more than once in subsequent steps. For example, "dop". Note: The order in which you list your letters matters!arrow_forwardFrom the table of available reagents select the one(s) you would use to accomplish the transformations shown below.arrow_forwardOrganic Chemistry: Alkyl halides only 1. For each of the following pairs of Sy2 reactions, indicate which reaction occurs faster: a) CH;CH,Br + H0 OR CH,CH,Br + OH b) Remember the following is under an S2 condition: CH,CHCH,Br CH3 + OH CH;CH2CHBR + OH OR ČH3 2. What is the product of the reaction of ethyl bromide with each of the following nucleophiles? a) CH,CH,CH,0 b) CH,C=C c) (CH,)N 3. Arrange the following alky halides in order of decreasing reactivity in an Sy1 reaction: a) 2-bromopentane, 2-chloropentane, 1-chloropentane, 3-bromo-3-methylpentanearrow_forward
- Draw the products of the reaction. Be sure to specify stereo- and regiochemistry when appropriatearrow_forwardMelting point: 98-105 Percent yield: 60% did you successfully make pure product? Explain.arrow_forwardThe second-order rate constant (in units of M-1s-1) for acid-catalyzed hydration at 25 °C is given for each of the following alkenes: a.Calculate the relative rates of hydration of the alkenes. (Hint: Divide each rate constant by the smallest rate constant of the series: 3.51 x 10-8.) b. Why does (Z)-2-butene react faster than (E)-2-butene? c. Why does 2-methyl-2-butene react faster than (Z)-2-butene? d. Why does 2,3-dimethyl-2-butene react faster than 2-methyl-2-butene?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Coenzymes and cofactors; Author: CH15 SWAYAM Prabha IIT Madras;https://www.youtube.com/watch?v=bubY2Nm7hVM;License: Standard YouTube License, CC-BY
Aromaticity and Huckel's Rule; Author: Professor Dave Explains;https://www.youtube.com/watch?v=7-BguH4_WBQ;License: Standard Youtube License