
Concept explainers
(a)
Interpretation:
The detailed mechanisms for the given reaction occurring via
Concept introduction:
The
In case of
Answer to Problem 8.44P
The
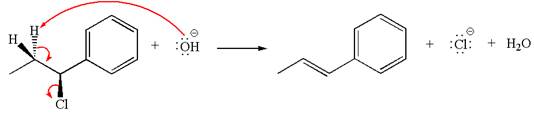
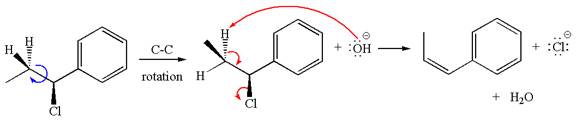
The
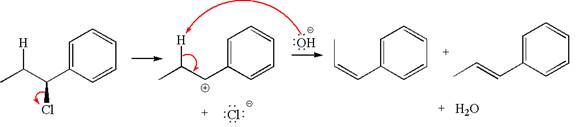
Explanation of Solution
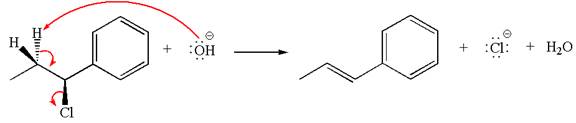
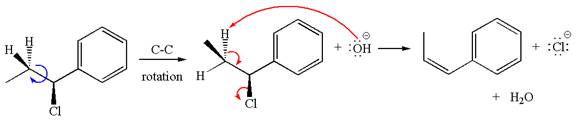
The given reaction equation is:
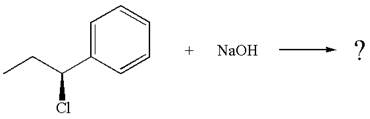
In the given reaction,
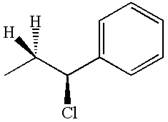
The hydrogen atom, indicated with the dash bond, is anti to
If the hydrogen atom, indicated with wedge bond, tends to eliminate, it must orient anti to
In
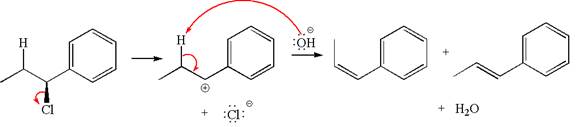
The products formed for the given reaction from both
(b)
Interpretation:
The detailed mechanisms for the given reaction occurring via
Concept introduction:
The
In case of
Answer to Problem 8.44P
The
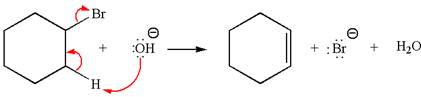
The
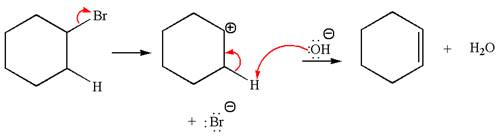
Explanation of Solution
The given reaction equation is:
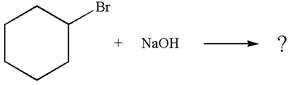
In the given reaction,
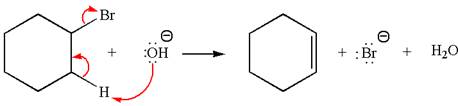
In
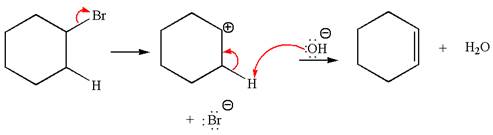
The products formed for the given reaction from both
(c)
Interpretation:
The detailed mechanisms for the given reaction occurring via
Concept introduction:
The
In case of
Answer to Problem 8.44P
The
The
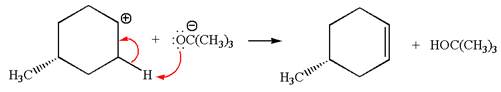
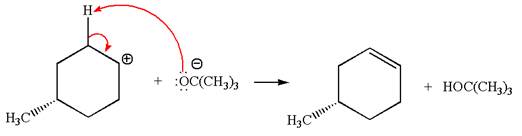
Explanation of Solution
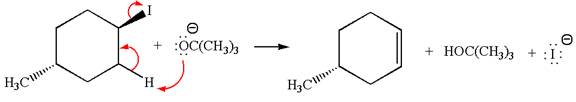
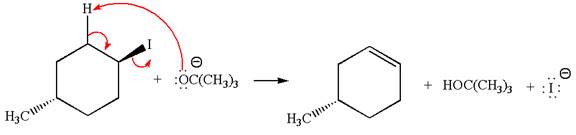
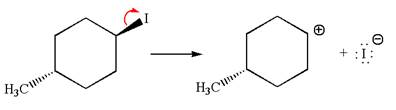
The given reaction equation is:
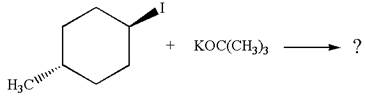
In the given reaction,
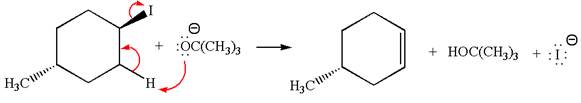
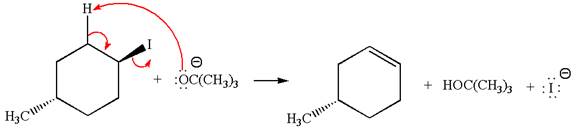
In
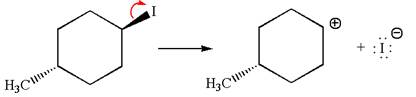
In the second step, the base abstracts the proton from the carbon adjacent to the positively charged carbon. Two products are possible because the proton gets eliminated from two different carbon atoms. The detailed mechanism is shown below:

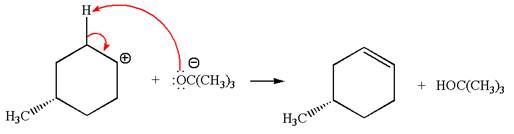
The products formed for the given reaction from both
(d)
Interpretation:
The detailed mechanisms for the given reaction occurring via
Concept introduction:
The
In case of
Answer to Problem 8.44P
The
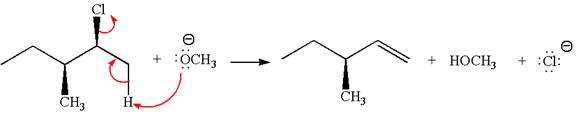
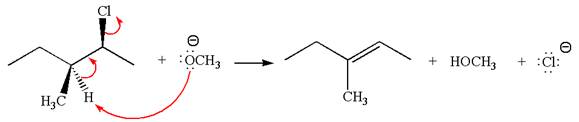
The
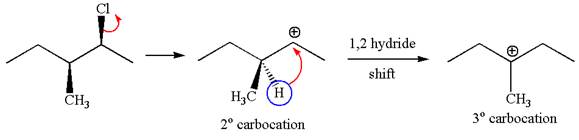
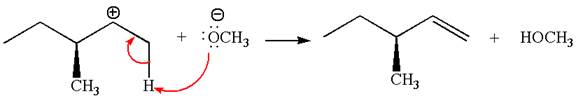
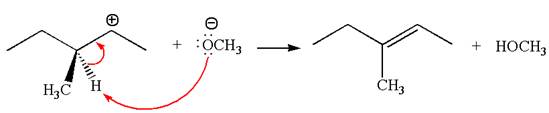
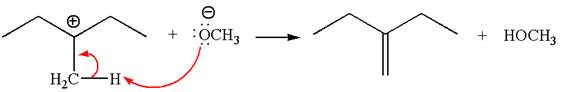
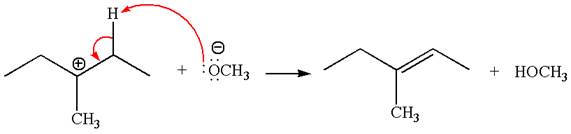
Explanation of Solution
The given reaction equation is:
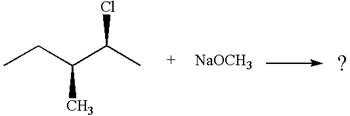
In the given reaction,
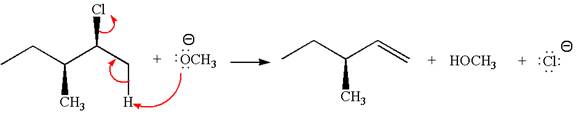
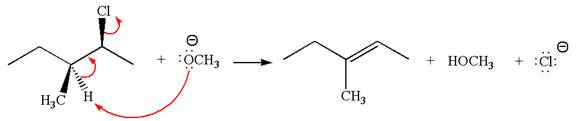
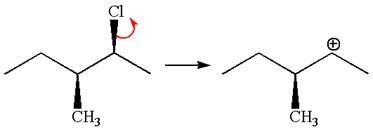
In
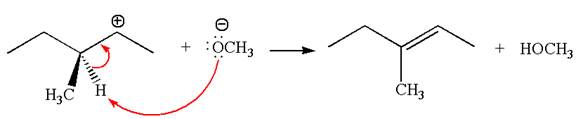
In the second step, the base abstracts the proton from the carbon adjacent to the positively charged carbon. Two products are possible from the secondary carbocation because the proton is eliminated from two different carbon atoms.

The carbocation formed is a secondary carbocation, which can be rearranged to a more stable tertiary carbocation by
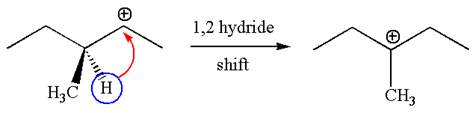
Two products are possible from the tertiary carbocation because the proton is eliminated from two different carbon atoms.
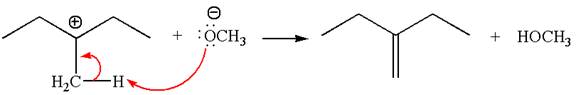
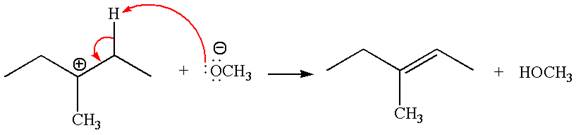
Thus, in all the reactions above, the products are formed by
The products formed for the given reaction from both
(e)
Interpretation:
The detailed mechanisms for the given reaction occurring via
Concept introduction:
The
In case of
Answer to Problem 8.44P
The
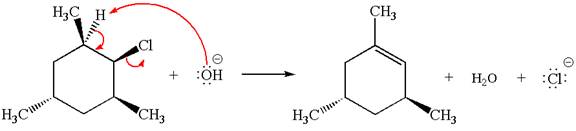
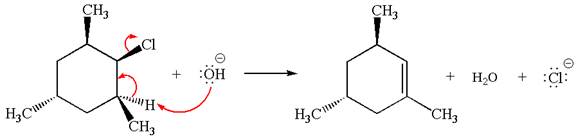
The
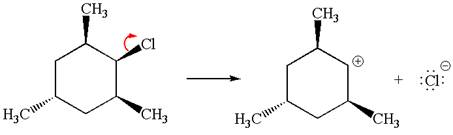
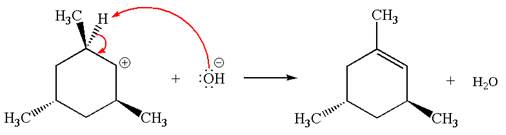
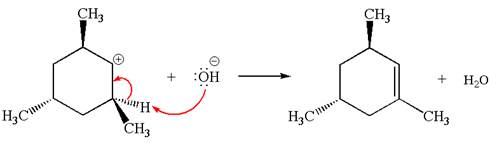
Explanation of Solution
The given reaction equation is:
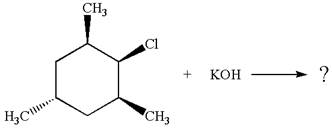
In the given reaction,
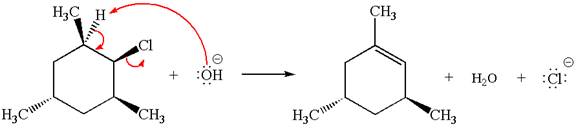

In

In the second step, the base abstracts the proton from the carbon adjacent to the positively charged carbon. Two products are possible because the proton is eliminated from two different carbon atoms. The detailed mechanism is shown below:


The products formed for the given reaction from both
(f)
Interpretation:
The detailed mechanisms for the given reaction occurring via
Concept introduction:
The
In case of
Answer to Problem 8.44P
The
The
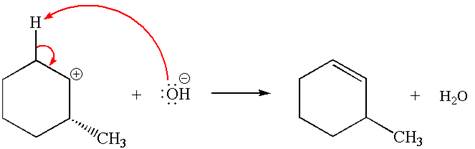
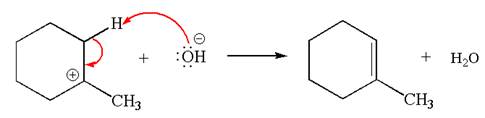
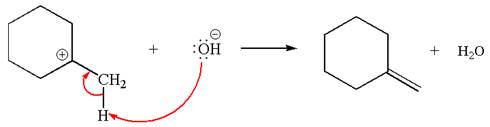
Explanation of Solution
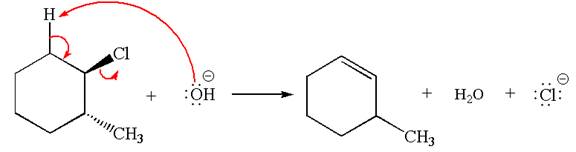
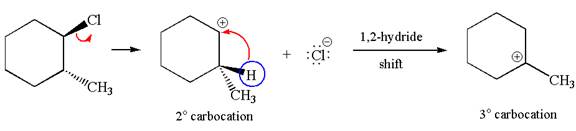
The given reaction equation is:
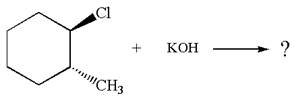
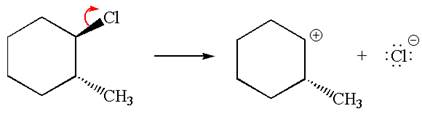
In the given reaction,
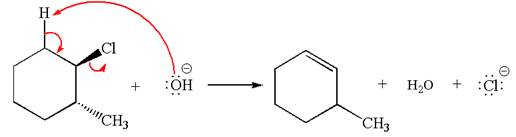
In
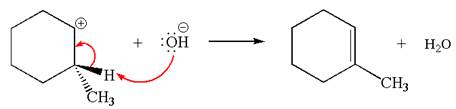
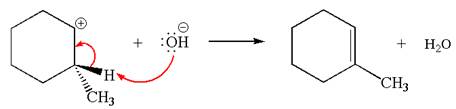
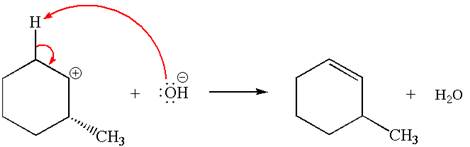
In the second step, the base abstracts the proton from the carbon adjacent to positively charged carbon. Two products are possible because the proton is eliminated from two different carbon atoms. The detailed mechanism is shown below:
The carbocation formed is a secondary carbocation, which can be rearranged to form more stable tertiary carbocation by
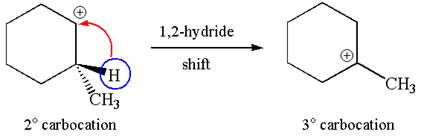
Two products are possible from the tertiary carbocation because the proton IS eliminated from two different carbon atoms.
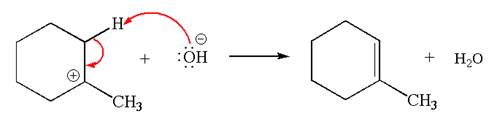
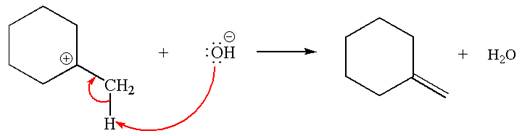
The products formed for the given reaction from both
Want to see more full solutions like this?
Chapter 8 Solutions
ORG CHEM W/ EBOOK & SW5 + STUDY GUIDE
- Draw the major organic product of the following reaction (see image), and select the mechanism which would dominate (SN1, SN2, E1, or E2).arrow_forwardDraw the complete mechanism for the following reaction. Predict the product(s) as well. Br₂arrow_forwardComplete the following E2 mechanism. You only need to draw the mechanism for oneof the products, but please draw both products that would be formed.arrow_forward
- Please show the products of following reactions and reaction mechanisms for each using the right arrows and define it as Sn2, Sn1, E2 or E1.Explain why do you choose that product and mechanism or in case something else happens explain why.arrow_forwardDraw a mechanism to account for the formation of the NaOH product in the reaction shown here. Hint: Under these A conditions, deprotonation of a propargylic (C=C-CH) carbon is reversible.arrow_forwardis this an E1 or E2 mechanism for this reaction? What is the major product and step by step mechanism?arrow_forward
- 3. When the following deuterium-labeled compound is treated with potassium tert-butoxide in DMF, a single product is observed. When the same substrate is heated in the presence of dilute potassium ethoxide in ethanol, a mixture of two products is formed. Provide the complete, detailed mechanism (curved arrows) for each reaction and label each reaction as E1 or E2. Note: deuterium is an isotope of hydrogen and can be treated similarly to hydrogen in chemical reactions but cannot be implied. H KOEt DMF ? D H dilute KOEt EtOH ?+ ?arrow_forwardFor each section, circle the mechanism from the two options given (E1 or E2) and draw the main organic product resulting from that mechanism. Indicate the stereochemistry and if two configurational isomers form, draw both.arrow_forwardThe transformation below takes place by two distinct reactions. Intermediate A is formed in the first reaction and then this goes on to the product in the second reaction. Provide a complete curved-arrow mechanism for all steps of both reactions.arrow_forward
- When the following deuterium-labeled compound is treated with potassium tert-butoxide in DMF, a single product is observed. When the same substrate is heated in the presence of dilute potassium ethoxide in ethanol, a mixture of two products is formed. Provide the complete, detailed mechanism (curved arrows) for each reaction and label each reaction as E1 or E2. Note: deuterium is an isotope of hydrogen and can be treated similarly to hydrogen in chemical reactions but cannot be implied. D H KO/Bu DMF D H dilute KOET EtOH ?+ ?arrow_forwardProvide the mechanism for each step of the given reaction, showing the major product as well.arrow_forwardDraw this compound in a chair form so that an E2 reaction is possible. Then, draw the mechanism and the major product. Your solution and structures must clearly show the 3D nature of the E2 mechanism.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
