
(a)
Interpretation:
The full set of possible quantum numbers for the outermost electron in
Concept introduction:
The electrons in the outermost occupied shell that determine the chemical properties of the elements are called the outermost electrons.
The quantum numbers provide complete information about the electron. There are four quantum numbers as follows:
1. The principal quantum number and it is represented by n. It tells about the shell to which the electron belongs.
2. The azimuthal quantum number and it is represented by l. It tells about the subshell of the electrons.
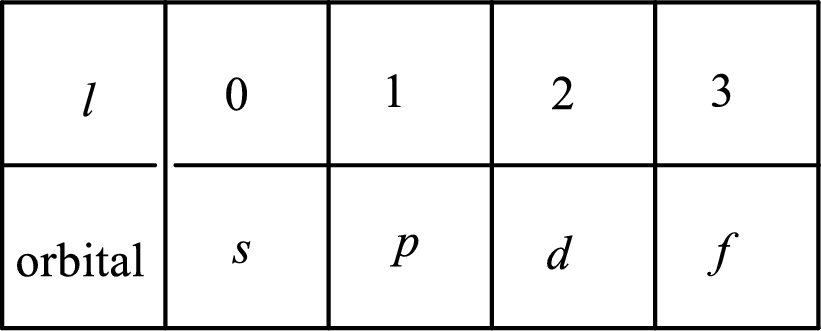
3. The magnetic quantum number and it is represented by
4. The spin quantum number and it is represented by
(a)
Answer to Problem 8.22P
The quantum numbers for the outermost electron in
Explanation of Solution
The
Its outermost electron enters in the
The value of the magnetic quantum number
The possible value of the spin quantum number
The quantum numbers for the outermost electron in
(b)
Interpretation:
The full set of possible quantum numbers for the electron gained when an
Concept introduction:
The electrons in the outermost occupied shell that determine the chemical properties of the elements are called the outermost electrons.
The quantum numbers provide complete information about the electron. There are four quantum numbers as follows:
1. The principal quantum number and it is represented by n. It tells about the shell to which the electron belongs.
2. The azimuthal quantum number and it is represented by l. It tells about the subshell of the electrons.
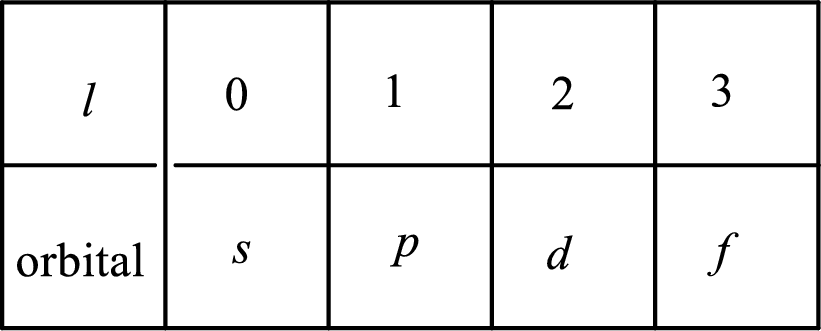
3. The magnetic quantum number and it is represented by
4. The spin quantum number and it is represented by
(b)
Answer to Problem 8.22P
The quantum numbers for the electron gained when an
Explanation of Solution
The atomic number of bromine is 35 so its electronic configuration is
Its outermost electron enters in the
The value of the magnetic quantum number
The possible value of the spin quantum number
The quantum numbers for the electron gained when an
(c)
Interpretation:
The full set of possible quantum numbers for the electron lost when a
Concept introduction:
The electrons in the outermost occupied shell that determine the chemical properties of the elements are called the outermost electrons.
The quantum numbers provide complete information about the electron. There are four quantum numbers as follows:
1. The principal quantum number and it is represented by n. It tells about the shell to which the electron belongs.
2. The azimuthal quantum number and it is represented by l. It tells about the subshell of the electrons.
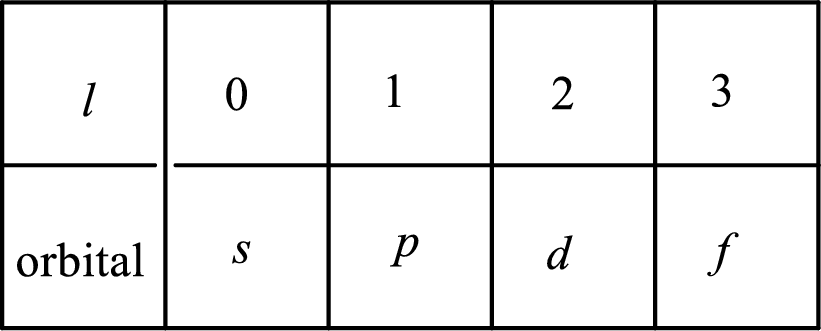
3. The magnetic quantum number and it is represented by
4. The spin quantum number and it is represented by
(c)
Answer to Problem 8.22P
The quantum numbers for the electron lost when gained when a
Explanation of Solution
The atomic number of cesium is 55 so its electronic configuration is
The ion formation occurs as:
The electron is lost from the
The quantum numbers for the electron lost when gained when a
(d)
Interpretation:
The full set of possible quantum numbers for the highest energy electron in the ground state of
Concept introduction:
The electrons in the outermost occupied shell that determine the chemical properties of the elements are called the outermost electrons.
The quantum numbers provide complete information about the electron. There are four quantum numbers as follows:
1. The principal quantum number and it is represented by n. It tells about the shell to which the electron belongs.
2. The azimuthal quantum number and it is represented by l. It tells about the subshell of the electrons.
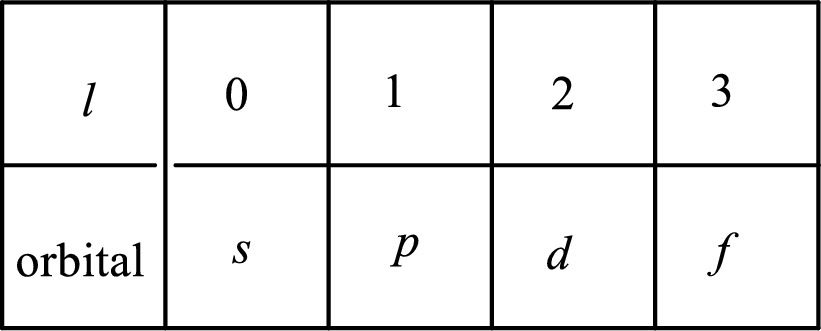
3. The magnetic quantum number and it is represented by
4. The spin quantum number and it is represented by
(d)
Answer to Problem 8.22P
The quantum numbers for the highest energy electron in the ground state of
Explanation of Solution
The atomic number of boron is 5 so its electronic configuration is
The electron is present in the
The value of the magnetic quantum number
The possible values of the spin quantum number
The quantum numbers for the highest energy electron in the ground state of
Want to see more full solutions like this?
Chapter 8 Solutions
MCGRAW: CHEMISTRY THE MOLECULAR NATURE
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





