
Concept explainers
(a)
Interpretation: The Lewis structure that contributes most to the bonding in
Concept introduction: The Lewis structures are diagrams that give information about the bonding electron pairs and the lone pairs of electrons in a molecule. Similar to electron dot structure in Lewis diagram the lone pair electrons are represented as dots and they also contain lines which represent bonding electron pairs in a bond.
To determine: If the given Lewis structure contributes to the bonding in
(a)
Answer to Problem 8.109QP
Solution
The given Lewis structure does not contribute most to the bonding in
Explanation of Solution
Explanation
The given Lewis structure is,
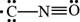
Figure 1
The charge present on each atom is known as formal charge which is calculated by using the formula,
For oxygen
The number of valence electrons in oxygen atom is six, the lone pair electrons are two and the bonding electrons are six.
Substitute the value of valence electrons, lone pair of electrons and bond pair of electrons in the above formula to calculate the formal charge.
For carbon atom,
The number of valence electrons in carbon atom is four, the lone pair electrons are five and the bonding electrons are two.
Substitute the value of valence electrons, lone pair of electrons and bond pair of electrons in the above formula to calculate the formal charge.
For nitrogen atom,
The number of valence electrons in nitrogen atom is five, the lone pair electron is zero and the bonding electrons are eight.
Substitute the value of valence electrons, lone pair of electrons and bond pair of electrons in the above formula to calculate the formal charge.
In the given Lewis structure of
(b)
To determine: If the given Lewis structure contributes to the bonding in
(b)
Answer to Problem 8.109QP
Solution
The given Lewis structure does not contribute most to the bonding in
Explanation of Solution
Explanation
The given Lewis structure is,
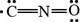
Figure 2
The formal charge is calculated by using the formula,
For oxygen atom,
The number of valence electrons in oxygen atom is six, the lone pair electrons are four and the bonding electrons are four.
Substitute the value of valence electrons, lone pair of electrons and bond pair of electrons in the above formula to calculate the formal charge.
For carbon atom,
The number of valence electrons in carbon atom is four, the lone pair electrons are three and the bonding electrons are four.
Substitute the value of valence electrons, lone pair of electrons and bond pair of electrons in the above formula to calculate the formal charge.
For nitrogen atom,
The number of valence electrons in nitrogen atom is five, the lone pair electron is zero and the bonding electrons are eight.
Substitute the value of valence electrons, lone pair of electrons and bond pair of electrons in the above formula to calculate the formal charge.
In the given Lewis structure of
Thus, it is known that the nitrogen atom is more electronegative than carbon atom. Hence, the given Lewis structure does not contribute most to the bonding in
(c)
To determine: If the given Lewis structure contributes to the bonding in
(c)
Answer to Problem 8.109QP
Solution
The given Lewis structure does not contribute most to the bonding in
Explanation of Solution
Explanation
The given Lewis structure is,
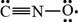
Figure 3
The formal charge is calculated by using the formula,
For oxygen atom,
The number of valence electrons in oxygen atom is six, the lone pair electrons are five and the bonding electrons are two.
Substitute the value of valence electrons, lone pair of electrons and bond pair of electrons in the above formula to calculate the formal charge.
For carbon atom,
The number of valence electrons in carbon atom is four, the lone pair electrons are two and the bonding electrons are six.
Substitute the value of valence electrons, lone pair of electrons and bond pair of electrons in the above formula to calculate the formal charge.
For nitrogen atom,
The number of valence electrons in nitrogen atom is five, the lone pair electron is zero and the bonding electrons are eight.
Substitute the value of valence electrons, lone pair of electrons and bond pair of electrons in the above formula to calculate the formal charge.
In the given Lewis structure of
Hence, the given Lewis structure does not contribute most to the bonding in
(d)
To determine: If the given Lewis structure contributes to the bonding in
(d)
Answer to Problem 8.109QP
Solution
The given Lewis structure contributes most to the bonding in
Explanation of Solution
Explanation
The given Lewis structure is,
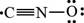
Figure 4
The formal charge is calculated by using the formula,
For oxygen atom,
The number of valence electrons in oxygen atom is six, the lone pair electrons are six and the bonding electrons are two.
Substitute the value of valence electrons, lone pair of electrons and bond pair of electrons in the above formula to calculate the formal charge.
For carbon atom,
The number of valence electrons in carbon atom is four, the lone pair electron is one and the bonding electrons are six.
Substitute the value of valence electrons, lone pair of electrons and bond pair of electrons in the above formula to calculate the formal charge.
For nitrogen atom,
The number of valence electrons in nitrogen atom is five, the lone pair electron is zero and the bonding electrons are eight.
Substitute the value of valence electrons, lone pair of electrons and bond pair of electrons in the above formula to calculate the formal charge.
In the given Lewis structure of
Conclusion
The Lewis structure that contributes most to the bonding in the
Want to see more full solutions like this?
Chapter 8 Solutions
Chemistry: The Science in Context (Fifth Edition)
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





