
Concept explainers
Interpretation:
Structures of the major organic products formed in the reaction of each of the given reagents with
Concept introduction:
Since
According to Markovnikov’s rule. when an unsymmetrically substituted alkene reacts with a hydrogen halide, hydrogen adds to the carbon that has the greater number of hydrogens and halogen adds to the carbon that has fewer hydrogens.
In a hydroboration oxidation reaction, hydrogen atom gets bonded to the carbon that has fewer hydrogens and the hydroxyl to the carbon that has a greater number of hydrogens. This is a rule opposite of Markovnikov’s addition.
Answer to Problem 29P
Solution:
a)
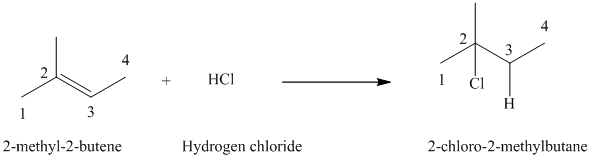
b)
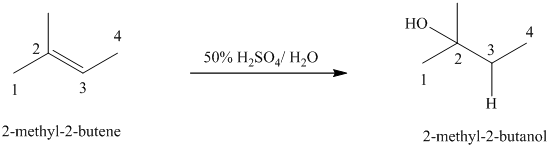
c)
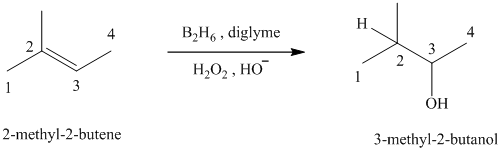
d)
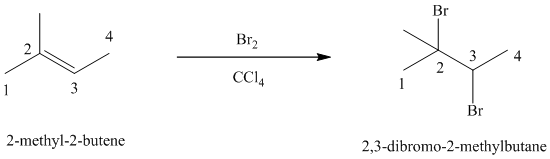
e)
f)
 g)
g)
h)
i)
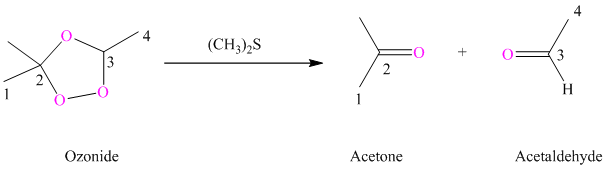
Explanation of Solution
a) The reaction is as follows:
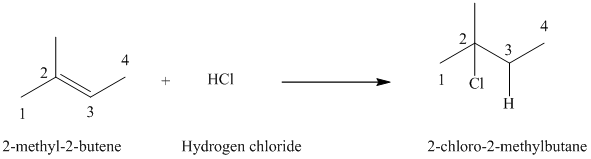
The given alkene
Hydrogen chloride adds to the double bond of
b) The reaction is as follows:
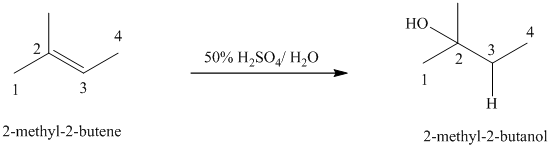
This reaction is an acid catalyzed electrophilic addition reaction of alkenes. In this reaction, water molecule gets added to the double bond in
In this reaction, the addition of water molecule to alkene follows Markovnikov’s rule. The hydrogen atom in water molecule adds to the carbon
The addition mechanism for this reaction follows Markovnikov’s rule, and so the major organic product for the above acid-catalyzed electrophilic addition reaction is
c) The reaction is as follows:
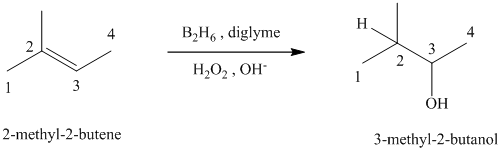
This reaction is the hydroboration-oxidation reaction of alkenes. In this reaction, water molecule gets added to the double bond in
The hydrogen atom from water molecule adds to the carbon
d) The reaction is as follows:
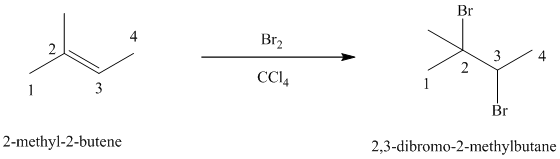
Bromine reacts rapidly with alkenes by electrophilic addition. The products are called vicinal dibromides, meaning that the bromine atoms are attached to adjacent double bonded carbon atoms. It is carried out in suitable solvents such as
Bromine adds across the double bond in
e) The reaction is as follows:
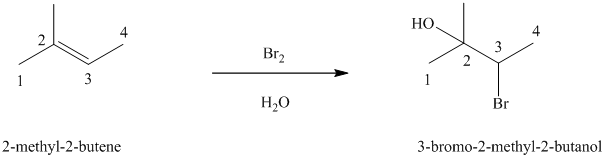
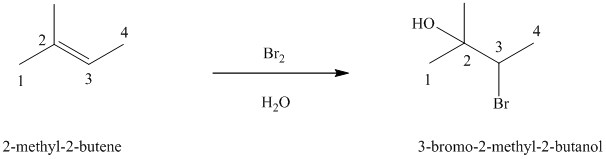
In aqueous solution, chlorine and bromine react with alkenes to give corresponding vicinal halohydrins. Halohydrin compounds have a halogen and hydroxyl group on adjacent carbon atoms. In alkene, halogen atom bonds to that carbon atom which has a larger number of hydrogens and hydroxyl group bonds to that carbon atom which has a smaller number of hydrogens.
In the reaction of
f) The reaction is as follows:
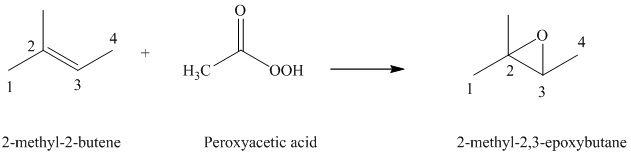
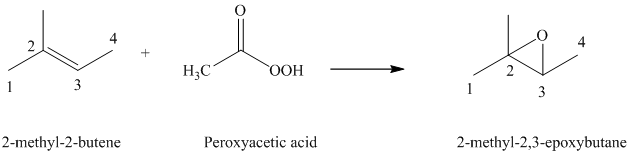
Peroxyacids transfers oxygen to the double bond of an alkene to yield epoxides. An epoxide is a three-membered oxygen-containing ring.
When
g) The reaction is as follows:
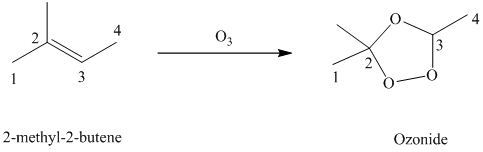
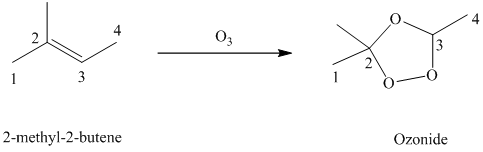
Ozone is a powerful electrophile and reacts with alkenes to cleave the double bond, forming an ozonide.
h) The reaction is as follows:
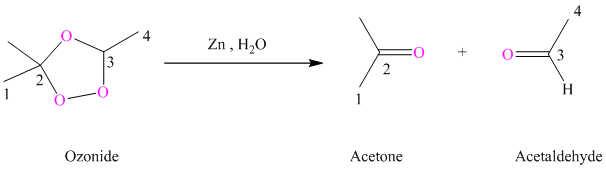
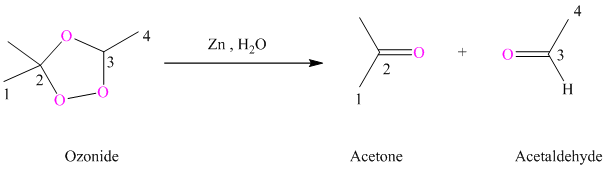
Ozonides are formed as a result of the reaction of ozone with an alkene. Ozonides undergo hydrolysis in the water giving carbonyl compounds. According to the structure of starting alkene, various carbonyl products are formed such as formaldehyde, aldehydes, or ketones.
When the corresponding ozonide of
i) The reaction is as follows:
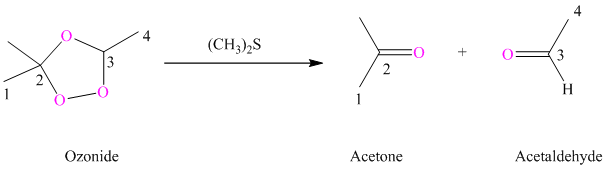
Ozonides are formed as a result of the reaction of ozone with an alkene. Ozonides undergo hydrolysis in water, giving carbonyl compounds. According to the structure of starting alkene, various carbonyl products are formed such as formaldehyde, aldehydes, or ketones.
When corresponding ozonide of
Want to see more full solutions like this?
Chapter 8 Solutions
ORGANIC CHEMISTRY-W/STUD.SOLN.MAN.
- Rank the compounds in each group according to their reactivity towardelectrophilic substitution.(a) Chlorobenzene, o-dichlorobenzene, benzene(b) p-Bromonitrobenzene , nitrobenzene, phenol(c) Fluorobenzene, benzaldehyde, a-xylene(d) Benzonitrile, p-methylbenzonitr ile,p-methoxybenzonitrilearrow_forwardExplain why :(a) The dipole moment of chlorobenzene is lower than that of cyclohexyl chloride.(b) Alkyl halides, though polar, are immiscible with water.arrow_forward(a) How will you carry out the following conversions?(i) Acetylene to Acetic acid (ii) Toluene to m-nitrobenzoic acid(iii) Ethanol to Acetone(b) Give reasons :(i) Chloroacetic acid is stronger than acetic acid.(ii) pH of reaction should be carefully controlled while preparing ammonia derivatives of carbonyl compounds.arrow_forward
- Provide the reagents and solvents (where appropriate) needed to bring about the following transformations. (a) CI (b)arrow_forward1. (a) Describe aromaticity, Kekule structure and resonance structure for benzene. (b) Why is benzene more stable than aliphatic alkenes?arrow_forwardWrite structural formulas for the cyclohexadienyl cations formed from aniline (C6H5NH2) during(a) Ortho bromination (four resonance structures)(b) Meta bromination (three resonance structures)(c) Para bromination (four resonance structures)arrow_forward
- (a) The Friedel-Crafts reaction of benzene with 2-chloro-3-methylbutane in the presence of AlCl3 occurs with a carbocation rearrangement. Give mechanistic explanation and the product formed. (b) Predict the product(s) will be formed from the following reactions: (i) Bromination of p-methylbenzoic acid (ii) Sulphonation of m-bromoanisole (iii) Friedel-craft acylation of o-bromonitrobenzenearrow_forwardThe hydrocarbon fluorene was treated with potassium t-butoxide in an acid-base reaction, giving the fluorenide anion and t-butyl alcohol. (a) Which way does the equilibrium lie, and by how much? b) What is the proportion of the fluorenide anion to fluorene? (c) Why is fluorene so highly acidic, considering the pKa of an average alkane is above 50?arrow_forwardStarting from bromoethane, the formation of which of the following compound requires more than one step of reaction? 2 (a) Methoxyethane (b) Ethanol (c) Ethanoic acid (d) Ethenearrow_forward
- Give the structure, exclusive of stereochemistry, of the principal organic product formed on reaction of 2,3-dimethyl-1,3-butadiene with each of the following:(a) 2 mol H2, platinum catalyst(b) 1 mol HCl (product of 1,2-addition)(c) 1 mol HCl (product of 1,4-addition)(d) 1 mol Br2 (product of 1,2-addition)(e) 1 mol Br2 (product of 1,4-addition)(f) 2 mol Br2arrow_forwardDescribe how would you distinguish the following pairs, (a) Benzene and cyclohexane (b) Phenol and toluene (c) Phenol and benzoic acidarrow_forward2. (a) Reaction of an alkene with ozone followed by an oxidative workup gives the product shown. What is the structure of alkene A? A (b) Compounds B and C both have the formula C10H16. Hydrogenation of either compound over Pt gives the same product, cis-1-isopropyl-4-methylcyclohexane. Ozonolysis with reductive workup fragments the two compounds differently, as shown below. What are the structures of C B and C? (CHCl3, chloroform, is the solvent.) B 1) 03, CHCI 3 2) (CH3)2S 1) 03, CHC13 2) (CH3)2S H O 1) 03 2) H₂O2, H₂O H3C- H O CH3 3. (a) Draw the structures of all enols that would spontaneously form this ketone, including stereoisomers. + + I HO₂C H + La CH3CH₂-C-CH(CH3)2 O= (b) Would alkyne hydration be a good preparative method for this compound? If so, give the reaction. If not, explain why.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





