
Organic Chemistry
5th Edition
ISBN: 9780078021558
Author: Janice Gorzynski Smith Dr.
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 7, Problem 7.55P
Draw the products of each
a. 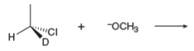 c.
c. 
b. 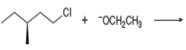
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Provide the correct common name for the compound shown here.
Ph
heat
heat
(12) Which one of the following statements about fluo-
rometry is FALSE?
a) Fluorescence is better detected at 90 from the exci-
tation direction.
b) Fluorescence is typically shifted to longer wave-
length from the excitation wavelength.
c) For most fluorescent compounds, radiation is pro-
duced by a
transition
Chapter 7 Solutions
Organic Chemistry
Ch. 7 - Problem 7.1 Telfairine, a naturally occurring...Ch. 7 - Give the IUPAC name for each compound. a. b. c. d.Ch. 7 - Prob. 7.3PCh. 7 - An sp3 hybridized CCl bond is more polar than an...Ch. 7 - Prob. 7.5PCh. 7 - Problem 7.6 Identify the nucleophile and leaving...Ch. 7 - Prob. 7.7PCh. 7 - Prob. 7.8PCh. 7 - What neutral nucleophile is needed to convert...Ch. 7 - Prob. 7.10P
Ch. 7 - Prob. 7.11PCh. 7 - Does the equilibrium favor the reactants or...Ch. 7 - Prob. 7.13PCh. 7 - Classify each solvent as protic or aprotic. a. b....Ch. 7 - Prob. 7.15PCh. 7 - Prob. 7.16PCh. 7 - Prob. 7.17PCh. 7 - Prob. 7.18PCh. 7 - Prob. 7.19PCh. 7 - Draw the product of each SN2 reaction and indicate...Ch. 7 - Prob. 7.21PCh. 7 - Prob. 7.22PCh. 7 - What happens to the rate of an SN1 reaction under...Ch. 7 - Draw the products of each SN1 reaction and...Ch. 7 - Classify each carbocation as 1,2, or 3. a. b. c....Ch. 7 - Prob. 7.26PCh. 7 - Prob. 7.27PCh. 7 - Prob. 7.28PCh. 7 - Prob. 7.29PCh. 7 - Problem 7.30 For each alkyl halide and...Ch. 7 - Prob. 7.31PCh. 7 - Prob. 7.32PCh. 7 - Prob. 7.33PCh. 7 - Prob. 7.34PCh. 7 - Prob. 7.35PCh. 7 - Prob. 7.36PCh. 7 - Prob. 7.37PCh. 7 - Prob. 7.38PCh. 7 - Prob. 7.39PCh. 7 - Give the IUPAC name for each compound, including...Ch. 7 - Draw the products formed when each alkyl halide is...Ch. 7 - Give the IUPAC name for each compound. a. c. e. b....Ch. 7 - Prob. 7.43PCh. 7 - Draw the eight constitutional isomers having the...Ch. 7 - Which compound in each pair has the higher boiling...Ch. 7 - Draw the products of each nucleophilic...Ch. 7 - Prob. 7.47PCh. 7 - Rank the species in each group in order of...Ch. 7 - Which of the following nucleophilic substitution...Ch. 7 - Rank the species in each group in order of...Ch. 7 - Prob. 7.51PCh. 7 - Prob. 7.52PCh. 7 - 7.53 Consider the following reaction.
Draw a...Ch. 7 - Prob. 7.54PCh. 7 - Draw the products of each SN2 reaction and...Ch. 7 - Prob. 7.56PCh. 7 - Prob. 7.57PCh. 7 - Consider the following SN1 reaction. a.Draw a...Ch. 7 - 7.59 Pick the reactant or solvent in each part...Ch. 7 - Draw the products of each SN1 reaction and...Ch. 7 - Prob. 7.61PCh. 7 - Prob. 7.62PCh. 7 - Prob. 7.63PCh. 7 - Fluticasone, the chapter-opening molecule, can be...Ch. 7 - Prob. 7.65PCh. 7 - 7.66 Diphenhydramine, the antihistamine in...Ch. 7 - Draw a stepwise, detailed mechanism for the...Ch. 7 - When a single compound contains both a nucleophile...Ch. 7 - Prob. 7.69PCh. 7 - Prob. 7.70PCh. 7 - Draw a stepwise, detailed mechanism f or the...Ch. 7 - Prob. 7.72PCh. 7 - Fill in the appropriate reagent or starting...Ch. 7 - Devise a synthesis of each compound from an alkyl...Ch. 7 - Suppose you have compounds A-D at y our disposal....Ch. 7 - Muscalure, the sex pheromone of the common...Ch. 7 - Prob. 7.77PCh. 7 - Prob. 7.78PCh. 7 - Draw a stepwise mechanism for the following...Ch. 7 - 7.80 As we will learn in Chapter 9, an epoxide is...Ch. 7 - Prob. 7.81PCh. 7 - In some nucleophilic substitutions under SN1...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Label each statement about the polynucleotide ATGGCG as true or false. The polynucleotide has six nucleotides. ...
General, Organic, and Biological Chemistry - 4th edition
Identify me theme or themes exemplified by (a) the sharp quills of a porcupine (b) the development of a multice...
Campbell Biology in Focus (2nd Edition)
Whether two metal foil leaves an electroscope get opposite charge when the electroscope is charged.
Physics of Everyday Phenomena
Separate the list P,F,V,,T,a,m,L,t, and V into intensive properties, extensive properties, and nonproperties.
Fundamentals Of Thermodynamics
2. A gene is a segment of DNA that has the information to produce a functional product. The functional product ...
Genetics: Analysis and Principles
Gregor Mendel never saw a gene, yet he concluded that some inherited factors were responsible for the patterns ...
Campbell Essential Biology (7th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Indicate the correct option.a) Graphite conducts electricity, being an isotropic materialb) Graphite is not a conductor of electricityc) Both are falsearrow_forward(f) SO: Best Lewis Structure 3 e group geometry:_ shape/molecular geometry:, (g) CF2CF2 Best Lewis Structure polarity: e group arrangement:_ shape/molecular geometry: (h) (NH4)2SO4 Best Lewis Structure polarity: e group arrangement: shape/molecular geometry: polarity: Sketch (with angles): Sketch (with angles): Sketch (with angles):arrow_forward1. Problem Set 3b Chem 141 For each of the following compounds draw the BEST Lewis Structure then sketch the molecule (showing bond angles). Identify (i) electron group geometry (ii) shape around EACH central atom (iii) whether the molecule is polar or non-polar (iv) (a) SeF4 Best Lewis Structure e group arrangement:_ shape/molecular geometry: polarity: (b) AsOBr3 Best Lewis Structure e group arrangement:_ shape/molecular geometry: polarity: Sketch (with angles): Sketch (with angles):arrow_forward
- (c) SOCI Best Lewis Structure 2 e group arrangement: shape/molecular geometry:_ (d) PCls Best Lewis Structure polarity: e group geometry:_ shape/molecular geometry:_ (e) Ba(BrO2): Best Lewis Structure polarity: e group arrangement: shape/molecular geometry: polarity: Sketch (with angles): Sketch (with angles): Sketch (with angles):arrow_forwardDon't used Ai solutionarrow_forwardDon't used Ai solutionarrow_forward
- reaction scheme for C39H4202 Hydrogenation of Alkyne (Alkyne to Alkene) show reaction (drawing) pleasearrow_forwardGive detailed mechanism Solution with explanation needed. Don't give Ai generated solutionarrow_forwardShow work with explanation needed....don't give Ai generated solutionarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Chapter 4 Alkanes and Cycloalkanes Lesson 2; Author: Linda Hanson;https://www.youtube.com/watch?v=AL_CM_Btef4;License: Standard YouTube License, CC-BY
Chapter 4 Alkanes and Cycloalkanes Lesson 1; Author: Linda Hanson;https://www.youtube.com/watch?v=PPIa6EHJMJw;License: Standard Youtube License