
A mixture containing 65.0 mole% acetone (Ac) and the balance acetic acid (AA) is separated in a continuous distillation column at 1 atm. A ?owchart for the operation is as follows:
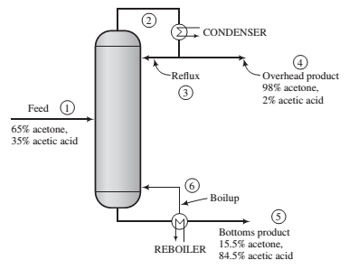
The stream from the top of the column is a vapor that passes though a condenser. The condensed liquid is divided into two equal streams: one is taken off as the overhead product (distillate) and the other (the re?ux) is returned to the column. The stream from the bottom of the column is a liquid that is partially vaporized in a reboiler. The liquid stream emerging from the reboiler is taken off as the bottoms product, and the vapor is returned to the column as boilup. Negligible heat is lost from the column, so that the only places in the system where external heat transfer takes place are the condenser and the reboiler.
|
|
||||
| Acetone | Acetic Acid | |||
| T(°C) |
|
|
|
|
| 56.8 | 0 | 7205 | 0 | 5723 |
| 63.0 | 205 | 7322 | 194 | 6807 |
| 67.5 | 354 | 7403 | 335 | 6884 |
| 98.7 | 1385 | 7946 | 1312 | 7420 |
- Taking 100 mol of feed as a basis, calculate the net heat requirement (cal) for the process. (You may neglect heats of mixing, although doing so for dissimilar liquids like acetone and acetic acid may introduce some error.)
- For the same basis, calculate the required heat input to the reboiler and the required heat removal from the condenser.
Want to see the full answer?
Check out a sample textbook solution
Chapter 7 Solutions
Elementary Principles of Chemical Processes, Binder Ready Version
Additional Engineering Textbook Solutions
INTERNATIONAL EDITION---Engineering Mechanics: Statics, 14th edition (SI unit)
Java: An Introduction to Problem Solving and Programming (8th Edition)
Elementary Surveying: An Introduction To Geomatics (15th Edition)
Thinking Like an Engineer: An Active Learning Approach (4th Edition)
Web Development and Design Foundations with HTML5 (8th Edition)
Starting Out with C++ from Control Structures to Objects (9th Edition)
- (f) SO: Best Lewis Structure 3 e group geometry:_ shape/molecular geometry:, (g) CF2CF2 Best Lewis Structure polarity: e group arrangement:_ shape/molecular geometry: (h) (NH4)2SO4 Best Lewis Structure polarity: e group arrangement: shape/molecular geometry: polarity: Sketch (with angles): Sketch (with angles): Sketch (with angles):arrow_forward1. Problem Set 3b Chem 141 For each of the following compounds draw the BEST Lewis Structure then sketch the molecule (showing bond angles). Identify (i) electron group geometry (ii) shape around EACH central atom (iii) whether the molecule is polar or non-polar (iv) (a) SeF4 Best Lewis Structure e group arrangement:_ shape/molecular geometry: polarity: (b) AsOBr3 Best Lewis Structure e group arrangement:_ shape/molecular geometry: polarity: Sketch (with angles): Sketch (with angles):arrow_forward(c) SOCI Best Lewis Structure 2 e group arrangement: shape/molecular geometry:_ (d) PCls Best Lewis Structure polarity: e group geometry:_ shape/molecular geometry:_ (e) Ba(BrO2): Best Lewis Structure polarity: e group arrangement: shape/molecular geometry: polarity: Sketch (with angles): Sketch (with angles): Sketch (with angles):arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





