
Organic Chemistry - Standalone book
10th Edition
ISBN: 9780073511214
Author: Francis A Carey Dr., Robert M. Giuliano
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 7, Problem 48P
The
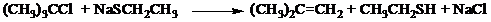
In the first order in
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
VI. The rate of the reaction
(CH3)3CCI + NaSCH2CH3 → (CH3)2C=CH2 + CH3CH2SH + NaCl
is first-order in (CH3)3CCI and first-order in NaSCH₂CH3. Please draw the rate-
limiting step of the reaction, using curved arrows to show the flow of electrons. If
any lone pairs are involved in a reaction, their involvement must be explicitly
shown. (
20
Draw the mechanism for the equilibrium illustrated below. What is the process called? Is the Keq >1, <1, =1 for this reaction as illustrated. Is the equilibrium direction dictated by thermodynamics or kinetics? Explain your rationale in detail.
The data below show the concentration of N2O5 versus time for the
following reaction:
N2O5 (g) → NO3 (g) + NO2(g)
Time (s) [N2O5] (M)
1.000
25
0.822
50
0.677
75
0.557
100
0.458
125
0.377
150
0.310
175
0.255
200
0.210
Chapter 7 Solutions
Organic Chemistry - Standalone book
Ch. 7.1 - Name each of the following using IUPAC...Ch. 7.1 - Prob. 2PCh. 7.2 - How many carbon atoms are sp2-hybridized in the...Ch. 7.3 - Prob. 4PCh. 7.3 - Are cis-2-hexene and trans-3-hexene stereoisomers?...Ch. 7.4 - Prob. 6PCh. 7.4 - Prob. 7PCh. 7.4 - Give the IUPAC name of each of the compounds in...Ch. 7.5 - Arrange the following in order of increasing...Ch. 7.6 - Prob. 10P
Ch. 7.6 - Standard enthalpies of formation are known for all...Ch. 7.6 - Prob. 12PCh. 7.6 - Despite numerous attempts, the alkene...Ch. 7.6 - Write structural formulas for the six isomeric...Ch. 7.7 - Place a double bond in the carbon skeleton shown...Ch. 7.9 - Identify the alkene obtained on dehydration of...Ch. 7.10 - Prob. 17PCh. 7.11 - Prob. 18PCh. 7.12 - Prob. 19PCh. 7.13 - The alkene mixture obtained on dehydration of...Ch. 7.14 - Write the structures of all the alkenes that can...Ch. 7.14 - Write structural formulas for all the alkenes that...Ch. 7.15 - A study of the hydrolysis behavior of...Ch. 7.15 - Use curved arrows to illustrate the electron flow...Ch. 7.15 - Predict the major product of the reaction shown.Ch. 7.16 - Prob. 26PCh. 7.17 - Prob. 27PCh. 7.18 - Prob. 28PCh. 7.19 - Predict the major organic product of each of the...Ch. 7.19 - A standard method for the synthesis of ethers is...Ch. 7 - Write structural formulas for each of the...Ch. 7 - Prob. 32PCh. 7 - Give an IUPAC name for each of the following...Ch. 7 - A hydrocarbon isolated from fish oil and from...Ch. 7 - Prob. 35PCh. 7 - Prob. 36PCh. 7 - Prob. 37PCh. 7 - Prob. 38PCh. 7 - Choose the more stable alkene in each of the...Ch. 7 - Suggest an explanation for the fact that...Ch. 7 - Prob. 41PCh. 7 - Write structural formulas for all the alkene...Ch. 7 - Prob. 43PCh. 7 - Prob. 44PCh. 7 - Predict the major organic product of each of the...Ch. 7 - Prob. 46PCh. 7 - Prob. 47PCh. 7 - The rate of the reaction In the first order in...Ch. 7 - Prob. 49PCh. 7 - Prob. 50PCh. 7 - You have available 2,2-dimethylcyclopentanol (A)...Ch. 7 - Prob. 52PCh. 7 - Prob. 53PCh. 7 - Prob. 54PCh. 7 - Acid-catalyzed dehydration of...Ch. 7 - The ratio of elimination to substitution is...Ch. 7 - Prob. 57PCh. 7 - Prob. 58DSPCh. 7 - Prob. 59DSPCh. 7 - Prob. 60DSPCh. 7 - Prob. 61DSPCh. 7 - A Mechanistic Preview of Addition Reactions The...Ch. 7 - Prob. 63DSPCh. 7 - Prob. 64DSPCh. 7 - Prob. 65DSP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Determine the reaction order for Na2S2O3 using calculations described in the background section. Show your work. Note that your answer will probably not be a whole number as it is in the examples.arrow_forwardThank you I did not connect ln 2 to 0.693 in the first order kinetic equation.arrow_forwardThere is great excess of H2O in this reaction.arrow_forward
- Predict the product of the following organic reaction: CH₂ CH O || C–CH2–CH=CH—(CH2)3 CH3 (CH₂)3 CH=CH- - CH₂ CH3 + 4 H₂ Ni CH,−O -C–CH2–CH=CH–CH=CH–CH2–CH3 Specifically, in the drawing area below, draw the chemical structure of the product P. If there is no product, because this reaction won't happen, check the No reaction box under the drawing area. Click and drag to start drawing a structure. × Ś +arrow_forward6 of 6 14. For the following first-order reaction X Y, the number of X molecules are placed in three same vessels at 100°C (i = 8 molecules, ii = 6 molecules, iii = 12 molecules) (a) compare rates of the reaction in these three vessels? (b) if the volume of each vessels were doubled, how would the relative rates be changed? (c) Explain the comparative t/2 of the reactions in (i) to (iii)? 15. Variation of the rate constant with temperature for the first-order reaction 2N2OS(g)→2N2O4ag) + Ozg) is given in the following table. Dctermine graphically the activation cnergy for the reaction. k (s) 1.74 x 105 T (K) 295 6.61 x 10 2.51 x 104 305 315 7.59 x 104 2.40 x 103 325 335arrow_forwardFind Delta H° for the overall reaction.arrow_forward
- What is equilibrium? Write the relationship between Kc and Kp for the reaction involving the gases such that, i. aA + bB ¤ cC + ɖD where A, B, C, and D are all gases, and a, b, c, and d are their respective coefficients, What is hybridization? Explain the hybridization in Phosphorus pentachloride (PCI5) molecule. iii. ii. What is rate law? Write the Integrated rate law expression for 1st order reaction. iv. What is a colligative property? Derive the expression for the elevation in boiling in point when a non-volatile solute is added to a solution. What is pH? Calculate the pH for 0.0001N NH4OH solution of 100mL volume. The pKb value for NH4OH at 25°C is 1.76 x 10-5. v.arrow_forward(a) When 3-bromo-2,3-dimethylpentane, (CH3)CH2CBr(CH3)CH2CH 3, reacts with aqueous potassiumhydroxide, an alcohol is formed.(i) Name the type of reaction taking place and give the role of the reagent.Type of reaction ...............................................................................................Role of reagent .................................................................................................(ii) Outline a mechanism for the reaction, showing clearly the structure of the alcohol formed. (b) When 3-bromo-2,3-dimethylpentane reacts with ethanolic potassium hydroxide, three structurallyisomeric alkenes are formed.(i) Name the type of reaction taking place and give the role of the reagent.Type of reaction ...............................................................................................Role of reagent .................................................................................................(ii) One of the reaction products is…arrow_forwardBond Bond energy (kcal/mol) B. Given in the table are the bond energies (in kcal/mol) of some of the bonds in the compounds involved in the free-radical chlorination of ethane. C-H 98 H-CI CI-CI 103 58 81 C-CI 1. Using these values calculate the AH of the two chain-propagating steps (steps 2 and 3). Show your calculations in the space provided below. AH step 2 ДН step 3arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Enzymes - Effect of cofactors on enzyme; Author: Tutorials Point (India) Ltd;https://www.youtube.com/watch?v=AkAbIwxyUs4;License: Standard YouTube License, CC-BY
Enzyme Catalysis Part-I; Author: NPTEL-NOC IITM;https://www.youtube.com/watch?v=aZE740JWZuQ;License: Standard Youtube License