
Concept explainers
(a)
Interpretation:
Four general properties of acidic solutions should be listed.
Concept Introduction:
The properties of acidic and basic solutions are different from each other because both are chemically opposite. Acidic and basic solutions together form water and salt. The reaction is known as neutralization reaction. In aqueous solution, acid gives hydrogen ion/s 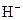 and base gives hydroxide ion/s
and base gives hydroxide ion/s 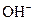 these positive and negative ions combined to form H2O and affect the concentration of aqueous solution.
these positive and negative ions combined to form H2O and affect the concentration of aqueous solution.
(b)
Interpretation:
Four general properties of basic solutions should be listed.
Concept Introduction:
The properties of acidic and basic solutions are different from each other because both are chemically opposite. Acidic and basic solutions together form water and salt. The reaction is known as neutralization reaction. In aqueous solution, acid gives hydrogen ion/s 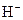 and base gives hydroxide ion/s
and base gives hydroxide ion/s 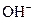 these positive and negative ions combined to form H2O and affect the concentration of aqueous solution.
these positive and negative ions combined to form H2O and affect the concentration of aqueous solution.
Want to see the full answer?
Check out a sample textbook solution
Chapter 7 Solutions
Chemistry For Changing Times (14th Edition)
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





