
Bundle: Chemistry: An Atoms First Approach, Loose-leaf Version, 2nd + OWLv2 with Student Solutions Manual, 4 terms (24 months) Printed Access Card
2nd Edition
ISBN: 9781337086431
Author: Steven S. Zumdahl, Susan A. Zumdahl
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 7, Problem 27E
Consider the following diagram when answering the questions below.
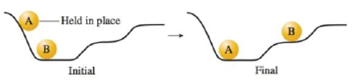
a. Compare balls A and B in terms of potential energy in both the initial and final setups.
b. Ball A has stopped moving in the figure on the right above, but energy must be conserved. What happened to the potential energy of ball A?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
An expression for the root mean square velocity, vrms, of a gas was derived. Using Maxwell’s velocity distribution, one can also calculate the mean velocity and the most probable velocity (mp) of a collection of molecules. The equations used for these two quantities are vmean=(8RT/πM)1/2 and vmp=(2RT/M)1/2 These values have a fixed relationship to each other.(a) Arrange these three quantities in order of increasing magnitude.(b) Show that the relative magnitudes are independent of the molar mass of the gas.(c) Use the smallest velocity as a reference for establishing the order of magnitude and determine the relationship between the larger and smaller values.
The reaction of solid dimethylhydrazine, (CH3)2N2H2, and liquefied dinitrogen tetroxide, N2O4, has been investigated for use as rocket fuel. The reaction produces the gases carbon dioxide (CO2), nitrogen (N2), and water vapor (H2O), which are ejected in the exhaust gases. In a controlled experiment, solid dimethylhydrazine was reacted with excess dinitrogen tetroxide, and the gases were collected in a closed balloon until a pressure of 2.50 atm and a temperature of 400.0 K were reached.(a) What are the partial pressures of CO2, N2, and H2O?(b) When the CO2 is removed by chemical reaction, what are the partial pressures of the remaining gases?
One liter of chlorine gas at 1 atm and 298 K reacts completely with 1.00 L of nitrogen gas and 2.00 L of oxygen gas at the same temperature and pressure. A single gaseous product is formed, which fills a 2.00 L flask at 1.00 atm and 298 K. Use this information to determine the following characteristics of the product:(a) its empirical formula;(b) its molecular formula;(c) the most favorable Lewis formula based on formal charge arguments (the central atom is N);(d) the shape of the molecule.
Chapter 7 Solutions
Bundle: Chemistry: An Atoms First Approach, Loose-leaf Version, 2nd + OWLv2 with Student Solutions Manual, 4 terms (24 months) Printed Access Card
Ch. 7 - Define the following terms: potential energy,...Ch. 7 - Consider the following potential energy diagrams...Ch. 7 - What is the first law of thermodynamics? How can a...Ch. 7 - When a gas expands, what is the sign of w? Why?...Ch. 7 - Prob. 5RQCh. 7 - High-quality audio amplifiers generate large...Ch. 7 - Explain how calorimetry works to calculate H or E...Ch. 7 - What is Hesss law? When a reaction is reversed,...Ch. 7 - Define the standard enthalpy of formation. What...Ch. 7 - Prob. 1ALQ
Ch. 7 - Prob. 2ALQCh. 7 - A fire is started in a fireplace by striking a...Ch. 7 - Liquid water turns to ice. Is this process...Ch. 7 - Prob. 5ALQCh. 7 - Prob. 6ALQCh. 7 - Consider 5.5 L of a gas at a pressure of 3.0 atm...Ch. 7 - Explain why oceanfront areas generally have...Ch. 7 - Hesss law is really just another statement of the...Ch. 7 - Prob. 10ALQCh. 7 - Prob. 11QCh. 7 - Prob. 12QCh. 7 - Assuming gasoline is pure C8H18(l), predict the...Ch. 7 - Prob. 14QCh. 7 - The enthalpy change for the reaction...Ch. 7 - For the reaction HgO(s)Hg(l)+12O2(g),H=+90.7KJ: a....Ch. 7 - Prob. 17QCh. 7 - The enthalpy change for a reaction is a state...Ch. 7 - Standard enthalpies of formation are relative...Ch. 7 - The combustion of methane can be represented as...Ch. 7 - Prob. 21QCh. 7 - Prob. 22QCh. 7 - Prob. 23QCh. 7 - Prob. 24QCh. 7 - Prob. 25ECh. 7 - Prob. 26ECh. 7 - Consider the following diagram when answering the...Ch. 7 - Consider the accompanying diagram. Ball A is...Ch. 7 - A gas absorbs 45 kJ of heat and does 29 kJ of...Ch. 7 - A system releases 125 kJ of heat while 104 kJ of...Ch. 7 - Calculate E for each of the following. a. q = 47...Ch. 7 - A system undergoes a process consisting of the...Ch. 7 - If the internal energy of a thermodynamic system...Ch. 7 - Calculate the internal energy change for each of...Ch. 7 - A sample of an ideal gas at 15.0 atm and 10.0 L is...Ch. 7 - Prob. 36ECh. 7 - Consider a mixture of air and gasoline vapor in a...Ch. 7 - As a system increases in volume, it absorbs 52.5 J...Ch. 7 - A balloon filled with 39.1 moles of helium has a...Ch. 7 - Prob. 40ECh. 7 - One of the components of polluted air is NO. It is...Ch. 7 - Prob. 42ECh. 7 - Are the following processes exothermic or...Ch. 7 - Are the following processes exothermic or...Ch. 7 - The overall reaction in a commercial heat pack can...Ch. 7 - Consider the following reaction:...Ch. 7 - Consider the combustion of propane:...Ch. 7 - Consider the following reaction:...Ch. 7 - Prob. 49ECh. 7 - The specific heat capacity of silver is 0.24 J/Cg....Ch. 7 - A 500-g sample of one of the substances listed in...Ch. 7 - Prob. 52ECh. 7 - A 30.0-g sample of water at 280. K is mixed with...Ch. 7 - A biology experiment requires the preparation of a...Ch. 7 - A 5.00-g sample of aluminum pellets (specific heat...Ch. 7 - Hydrogen gives off 120. J/g of energy when burned...Ch. 7 - Prob. 57ECh. 7 - A 110.-g sample of copper (specific heat capacity...Ch. 7 - In a coffee-cup calorimeter, 50.0 mL of 0.100 M...Ch. 7 - In a coffee-cup calorimeter, 100.0 mL of 1.0 M...Ch. 7 - A coffee-cup calorimeter initially contains 125 g...Ch. 7 - In a coffee-cup calorimeter, 1.60 g NH4NO3 is...Ch. 7 - Consider the dissolution of CaCl2:...Ch. 7 - Consider the reaction...Ch. 7 - The heat capacity of a bomb calorimeter was...Ch. 7 - The combustion of 0.1584 g benzoic acid increases...Ch. 7 - The enthalpy of combustion of solid carbon to form...Ch. 7 - Combustion reactions involve reacting a substance...Ch. 7 - Given the following data calculate H for the...Ch. 7 - Given the following data...Ch. 7 - Prob. 71ECh. 7 - Calculate H for the reaction...Ch. 7 - Given the following data...Ch. 7 - Given the following data...Ch. 7 - Give the definition of the standard enthalpy of...Ch. 7 - Write reactions for which the enthalpy change will...Ch. 7 - Prob. 77ECh. 7 - Use the values of Hf in Appendix 4 to calculate H...Ch. 7 - The Ostwald process for the commercial production...Ch. 7 - Calculate H for each of the following reactions...Ch. 7 - The reusable booster rockets of the space shuttle...Ch. 7 - The space shuttle Orbiter utilizes the oxidation...Ch. 7 - Consider the reaction...Ch. 7 - The standard enthalpy of combustion of ethene gas,...Ch. 7 - Water gas is produced from the reaction of steam...Ch. 7 - Prob. 86ECh. 7 - Prob. 87ECh. 7 - Prob. 88ECh. 7 - Some automobiles and buses have been equipped to...Ch. 7 - The complete combustion of acetylene, C2H2(g),...Ch. 7 - Prob. 91AECh. 7 - One way to lose weight is to exercise! Walking...Ch. 7 - Three gas-phase reactions were run in a...Ch. 7 - Nitrogen gas reacts with hydrogen gas to form...Ch. 7 - Combustion of table sugar produces CO2(g) and H2O(...Ch. 7 - Prob. 96AECh. 7 - Consider the following cyclic process carried out...Ch. 7 - Calculate H for the reaction...Ch. 7 - The enthalpy of neutralization for the reaction of...Ch. 7 - Prob. 100AECh. 7 - If a student performs an endothermic reaction in a...Ch. 7 - In a bomb calorimeter, the reaction vessel is...Ch. 7 - The bomb calorimeter in Exercise 102 is filled...Ch. 7 - Prob. 104AECh. 7 - Consider the following equations:...Ch. 7 - Prob. 106AECh. 7 - At 298 K, the standard enthalpies of formation for...Ch. 7 - Prob. 108AECh. 7 - A sample of nickel is heated to 99.8C and placed...Ch. 7 - Quinone is an important type of molecule that is...Ch. 7 - Calculate H for each of the following reactions,...Ch. 7 - Compare your answers from parts a and b of...Ch. 7 - Compare your answer from Exercise 72 of Chapter 3...Ch. 7 - Consider a balloon filled with helium at the...Ch. 7 - Prob. 115CWPCh. 7 - Prob. 116CWPCh. 7 - Prob. 117CWPCh. 7 - A swimming pool, 10.0 m by 4.0 m, is filled with...Ch. 7 - Prob. 119CWPCh. 7 - Calculate H for the reaction...Ch. 7 - Which of the following substances have an enthalpy...Ch. 7 - Consider 2.00 moles of an ideal gas that are taken...Ch. 7 - For the process H2O(l)H2O(g) at 298 K and 1.0 atm,...Ch. 7 - The sun supplies energy at a rate of about 1.0...Ch. 7 - Prob. 125CPCh. 7 - The standard enthalpies of formation for S(g),...Ch. 7 - Use the following standard enthalpies of formation...Ch. 7 - The standard enthalpy of formation for N2H4(g) is...Ch. 7 - The standard enthalpy of formation for NO(g) is...Ch. 7 - A piece of chocolate cake contains about 400...Ch. 7 - You have a l.00-mole sample of water at 30.C and...Ch. 7 - A 500.0-g sample of an element at 195C is dropped...Ch. 7 - A cubic piece of uranium metal (specific heat...Ch. 7 - On Easter Sunday, April 3, 1983, nitric acid...Ch. 7 - Using data from Chapter 2, calculate the change in...Ch. 7 - In Exercise 89 in Chapter 3, the Lewis structures...Ch. 7 - A gaseous hydrocarbon reacts completely with...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- How does the square root mean square velocity of gas molecules vary with temperature? Illustrate this relationship by plotting the square root mean square velocity of N2 molecules as a function of temperature from T=100 K to T=300 K.arrow_forwardDraw product B, indicating what type of reaction occurs. F3C CF3 NH2 Me O .N. + B OMearrow_forwardBenzimidazole E. State its formula. sState the differences in the formula with other benzimidazoles.arrow_forward
- Draw product A, indicating what type of reaction occurs. F3C CN CF3 K2CO3, DMSO, H₂O2 Aarrow_forward19) Which metal is most commonly used in galvanization to protect steel structures from oxidation? Lead a. b. Tin C. Nickel d. Zinc 20) The following molecule is an example of a: R₁ R2- -N-R3 a. Secondary amine b. Secondary amide c. Tertiary amine d. Tertiary amidearrow_forwardpls helparrow_forward
- pls helparrow_forward35) Complete the following equation by drawing the line the structure of the products that are formed. Please note that in some cases more than one product is possible. You must draw all possible products to recive full marks! a. ethanol + 2-propanol + H2SO4 → b. OH conc. H2SO4 CH2 H3C CH + K2Cr2O7 C. d. H3C A pressure CH3 + H2 CH Pt catalystarrow_forward21) The rate of reaction depends upon: a. the concentration and nature of reactants b. the temperature of the reaction C. whether or not a catalyst was used d. all of the above 22) A Maxwell-Boltzmann curve shows the distribution of molecular energies in a reaction system. When the temperature in this system is increased, the peak is a. higher and further to the right. b. higher and further to the left. c. lower and further to the right. d. lower and further to the left. 23) Which of the following correctly describes the reaction represented by the reaction below? CaCO3 (s) + energy → CaO (s) + CO2 (g) a. It is exothermic and the potential energy is greater in the reactants than the products. b. c. It is exothermic and the potential energy is greater in the products than the reactants. It is endothermic and the potential energy is greater in the products than the reactants. d. It is endothermic and the potential energy is equal for the products and reactants.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div
World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning


Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning

World of Chemistry
Chemistry
ISBN:9780618562763
Author:Steven S. Zumdahl
Publisher:Houghton Mifflin College Div

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning
The Laws of Thermodynamics, Entropy, and Gibbs Free Energy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=8N1BxHgsoOw;License: Standard YouTube License, CC-BY