
Concept explainers
To explain: The reason behind the sugar alcohol (monosaccharide derivatives) is no longer designated as D or L.
Introduction:
Monosaccharide having five carbons is known as pentose. The molecular formula of carbohydrate is Cn(H2O)n where n represents a whole number like 3 or higher. Monosaccharide contains various functional groups like alcohol,
Answer to Problem 1P
Answer: Fig.1 represents the structure of glyceraldehyde.
Pictorial representation: Fig.1 represents the structure of glyceraldehyde.
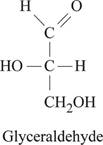
Fig.1: Structure of glyceraldehyde.
Explanation of Solution
Explanation:
Glyceraldehyde belongs to a group of monosaccharide and chemical formula for glyceraldehyde is C3H6O3. The carbonyl group present at 1st position of glyceraldehyde gets reduced into hydroxyl group. This reduction of carbonyl group results in the formation of glycerol structure. Fig.1 represents the structure of glyceraldehyde.
If the central carbon atom of a compound is bonded with four different functional groups, then the compound is said to be non-super imposable. On the basis of the arrangement of H and OH group present in chiral carbon atom, the compound is designated as D or L forms. L-form of glyceraldehyde has the -OH groups at the chiral center present far from carbonyl on the left side of the molecule. In D-form, glyceraldehyde has the -OH groups at the chiral center present far from carbonyl on the right side of the molecule. In glycerol, there is no chiral carbon is present. Thus, the sugar alcohol is no longer designated as D or L-form.
Want to see more full solutions like this?
Chapter 7 Solutions
Lehninger Principles of Biochemistry
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON





