
Concept explainers
Interpretation:
Reagent for the given conversion has to be predicted.
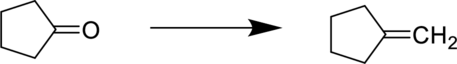
Concept Introduction:
Wittig Reaction: It is an organic reaction where an
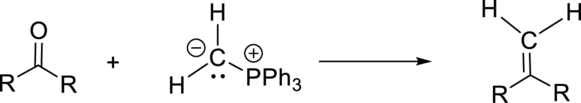
Non-stabilized ylide (having electron donating group on negatively charged carbon) gives Z-isomer whereas stabilized ylide (having electron withdrawing group on negatively charged carbon) gives E-isomer.
Explanation of Solution
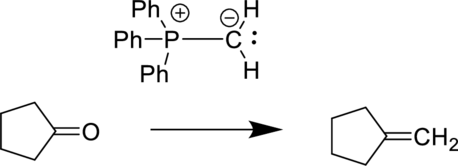
The reaction is shown below:
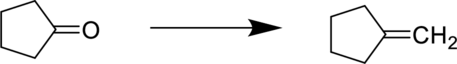
The starting material is a ketone and the product is an alkene. In-order to do this conversion, Wittig reagent can be used.
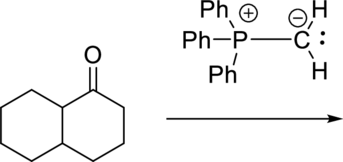
The given reactant is a ketone and a simple phosphorus ylide is the reagent. The given reagent is generally known as Wittig reagent and it is used to convert ketone functional group to alkene.
The reaction is given below:
Want to see more full solutions like this?
Chapter 6 Solutions
Organic Chemistry As a Second Language: Second Semester Topics
- Show all your work for the mechanism below using catalytic reformation. Hexane to methylcyclopentane to benzene.arrow_forwardYou continue to impress people at the chemical company and they give you a special assignment. Compound C needs to be converted to Compound D and you are tasked to come up with a route for this. Design a route to take C to D, outlining the reagents you would use for each step. You should use only reactions that have been covered in the Oranic chem 1 course, and it will take more than one steparrow_forwardConsider the reactions below and correctly PREDICT the products in the boxes below:arrow_forward
- Q: Provide the reagents needed to carry out the reactions below (or the product obtained). If two steps are needed, clearly indicate 1. (reagent A); 2. (reagent B). If separate steps are not indicated, I will assume you mean that all reagents are introduced together. Will H20 and H30+ work if so can you explain how for this question I am working on 2Earrow_forwardThis is a problem I am struggling with, because I still struggle to understand how to accurately describe why the nitro benzene-type structure is difficult to synthesize. Please help! Also the second problem I am really struggling to put words into a problem in order to solve it. Please show all steps for both 1 and 2!arrow_forwardHello, I hope you are doing well on this fine day. For the following quetion please read carefully the question and instruction. PLEASE ANSWER QUESTION IN 20 MINTUES NOT MORE PLEASE AND THANK YOU. If you do answer the question correctly and post it in the next 15 minutes, NO NEED TO SHOW THE WORK, I JUST WOULD LIKE THE CORRECT ANSWER AS SOON AS POSSIBLE. I will write a wonderful and generous feedback/review/rating about you. If you react 4.3 ml of ethanol with 7 drops of sulfuric acid and 0.227 g of salicylic acid, what is the theoretical yield of ethyl salicylate in grams (g)? The molecular weight of salicylic acid is 138.12 g/mol, the molecular weight of ethyl salicylate is 166.17 g/mol. The molecular weight of ethanol is 46.069 g/mol and its density is 0.789 g/mL. The molarity of sulfuric acid is 18 mol/Larrow_forward
- In the reaction series below, write down the appropriate reagents that can be used where there are question marks.arrow_forwardUsing your reaction roadmap as a guide, show how to convert ethylene into 1-butene. All of the carbon atoms of the target molecule must be derived from ethylene. Show all intermediate molecules synthesized along the way.arrow_forwardPlease help me with the organic chemistry problems below - they are based on predicting the product.arrow_forward
- Have this multistep condensation reaction series for practice -- any assistance in understanding the mechanisms and products would be greatly appreciated. Thanks!arrow_forwardWhat are all three products that can result from this E1 mechanism? (1 major, 2 minor)?arrow_forwardRead these directions carefully. For the reaction of 3-methyl-1-propene with Cl2 and H2O shown below, fill in the details of the mechanism. Draw the appropriate chemical structures and use an arrow to show how pairs of electrons are moved to make and break bonds during the reaction. Be sure to write all lone pairs of electrons and all formal charges. Finally, in the boxes provided by the arrows, write which kind of mechanistic element is being indicated, such as "make a bond", "add a proton", etc.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

