
Concept explainers
Interpretation:
The most stable chair conformation for the given compound has to be drawn.
Concept Introduction:
When a compound contains a cyclohexane ring in it, then there are three possibilities of conformations. They are chair, boat and twist-boat. Among these three conformations of cyclohexane ring, the most stable one is the chair conformation.

In the above structure, there are twelve substituents that can be placed. Among the twelve, six are axial substituents and six are equatorial substituents. The axial substituents can be represented as shown below,
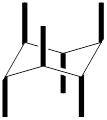
The equatorial substituents can be represented as,
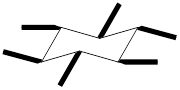
To draw a group in a correct configuration, we have to understand what is coming towards us and what is going away from us (wedge and dash bonds).
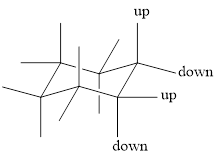
For drawing the other chair conformation, a ring flip has to be done. Ring flipping makes the skeleton as shown below,

During this ring flip from one chair conformation to another, all the substituents present in axial position goes to equatorial position and vice-versa.
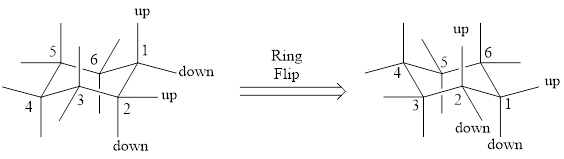
The most stable conformation among the two chair conformations can be found by looking into the bulky groups that are present in the ring. The chair conformation which has the bulky group in equatorial position will be the more stable one as there wont be steric interaction. If the bulky group is present in axial position, then there will be axial-axial interaction, which makes the conformation less stable. In simple words, we can say that the bulky group will prefer to be in equatorial position.
Want to see the full answer?
Check out a sample textbook solution
Chapter 6 Solutions
Organic Chemistry As a Second Language: First Semester Topics
- this is an organic chemistry question please answer accordindly!! please post the solution draw the figures on a paper please hand drawn and post, please answer EACH part till the end and dont just provide wordy explanations, please draw them on a paper and post clearly!! answer the full question with all details EACH PART CLEARLY please thanks!! im reposting this please solve all parts and draw it not just word explanations!!arrow_forwardA mixture of 0.412 M C12, 0.544 M F2, and 0.843 M CIF is enclosed in a vessel and heated to 2500 K. C12(g) + F2(g )2CIF(g) Kc = 20.0 at 2500 K Calculate the equilibrium concentration of each gas at 2500 K. [C12] = M [F2] = M [ CIF] =arrow_forwardShow reaction mechanism with explanation. don't give Ai generated solutionarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





