
A heat pump with refrigerant-134a as the working fluid is used to keep a space at 25°C by absorbing heat from geothermal water that enters the evaporator at 60°C at a rate of 0.065 kg/s and leaves at 40°C. Refrigerant enters the evaporator at 12°C with a quality of 15 percent and leaves at the same pressure as saturated vapor. If the compressor consumes 1.6 kW of power, determine (a) the mass flow rate of the refrigerant, (b) the rate of heat supply, (c) the COP, and (d) the minimum power input to the compressor for the same rate of heat supply.
FIGURE P6–152
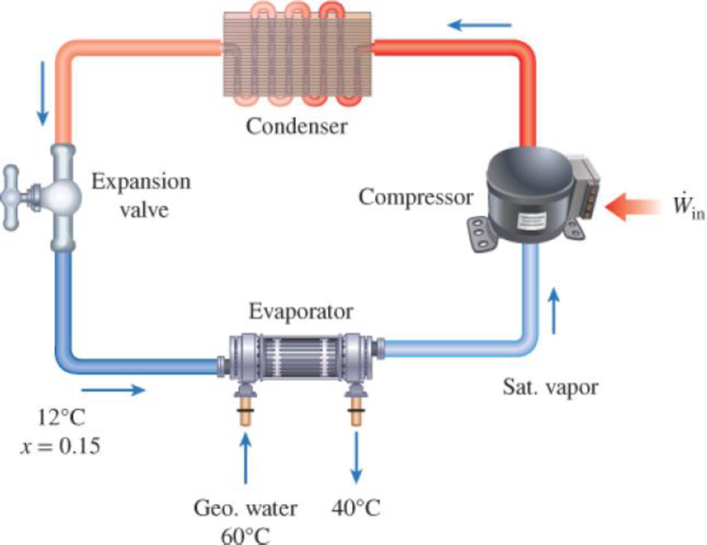
(a)
The mass flow rate of the refrigerant.
Answer to Problem 149RP
The mass flow rate of the refrigerant is
Explanation of Solution
Determine the rate of heat absorbed from the water.
Here, the mass flow rate of the water is
Determine the mass flow rate of a refrigerant.
Conclusion:
From the Table A-11, “Saturated refrigerant R-134a”, obtain the value of saturated pressure of the refrigerant at the inlet temperature of
Here, the pressure of refrigerant is constant in evaporation.
From the Table A-11, “Saturated refrigerant R-134a” to obtain the value of specific enthalpy of the refrigerant at the outlet pressure of
From the Table A-11, “Saturated refrigerant R-134a” to obtain the value of specific enthalpy of saturated liquid and specific enthalpy change upon vaporization of the refrigerant at the inlet temperature of
Calculate the specific enthalpy of refrigerant at evaporator inlet.
Here, the specific enthalpy of saturated liquid is
Substitute
From the Table A-4, “Saturated water-temperature” to obtain the value of specific enthalpy of saturated liquid of water at the inlet temperature of
From the Table A-4, “Saturated water-temperature” to obtain the value of specific enthalpy of saturated liquid of water at the outlet temperature of
Substitute
Substitute
Thus, the mass flow rate of the refrigerant is
(b)
The heating load of the heat pump.
Answer to Problem 149RP
The heating load of the heat pump is
Explanation of Solution
Determine the heating load of the heat pump.
Here, the power input consumed by compressor is
Conclusion:
Substitute
Thus, the heating load of the heat pump is
(c)
The COP of a heat pump operating between the same temperature limits.
Answer to Problem 149RP
The COP of a heat pump operating between the same temperature limits is
Explanation of Solution
Determine the coefficient of performance of the heat pump.
Conclusion:
Substitute
Thus, the COP of a heat pump operating between the same temperature limits is
(d)
The minimum power input to the compressor.
Answer to Problem 149RP
The minimum power input to the compressor is
Explanation of Solution
Determine the maximum coefficient of performance of the heat pump operating between the same temperature limits.
Here, the temperature of higher temperature body is
Determine the minimum power input to the condenser for the same heat pump load.
Conclusion:
Substitute
Substitute
Thus, the minimum power input to the compressor is
Want to see more full solutions like this?
Chapter 6 Solutions
Thermodynamics: An Engineering Approach
- A heat pump with refrigerant-134a as the working fluid is used to keep a space at 25°C by absorbing heat from geothermal water that enters the evaporator at 60°C at a rate of 0.065 kg/s and leaves at 40°C. Refrigerant enters the evaporator at 12°C with a quality of 15 percent and leaves at the same pressure as saturated vapor. If the compressor consumes 1.6 kW of power, determine the minimum power input to the compressor for the same rate of heat supply.arrow_forwardA heat pump with refrigerant-134a as the working fluid is used to keep a space at 25°C by absorbing heat from geothermal water that enters the evaporator at 60°C at a rate of 0.065 kg/s and leaves at 40°C. Refrigerant enters the evaporator at 12°C with a quality of 15 percent and leaves at the same pressure as saturated vapor. If the compressor consumes 1.6 kW of power, determine the COP.arrow_forwardA heat pump with refrigerant-134a as the working fluid is used to keep a space at 25°C by absorbing heat from geothermal water that enters the evaporator at 60°C at a rate of 0.065 kg/s and leaves at 40°C. Refrigerant enters the evaporator at 12°C with a quality of 15 percent and leaves at the same pressure as saturated vapor. If the compressor consumes 1.6 kW of power, determine the rate of heat supply.arrow_forward
- The two-stage compression refrigeration system shown below is used to remove heat from refrigerated space using R-410a as the coolant. The R-410a leaves the evaporator at state 1 and is first compressed in a low-pressure compressor (W, Pc=-250 kW) to an intermediate pressure of 933.9 kPa before it is mixed with saturated vapor and then further compressed in a high-pressure compressor to 3000 kPa. Heat is then removed from the R-410a as it passes through the heat exchanger and exchanges heat with cooling water. The R-410a is then expanded in the throttling valve to the intermediate pressure and passed through a type of mixing chamber called a flash chamber where it is separated into a saturated vapor leaving at state 7 and a saturated liquid leaving at state 8. Finally, the liquid is expanded before entering the evaporator at state 9. Note that the mass flow rate of R-410a leaving the mixing chamber is mypC = 10 kg/s. Neglect changes in kinetic and potential energy across all devices…arrow_forwardA vacuum refrigeration system consists of a large insulated flash chamber kept at low pressure by steam ejector which pumps vapor to a condenser. Condensate is removed by condensate to an air vent. Warm return water enters the flash chamber at 13oC, chilled water comes out of the flash chamber at 5oC Vapor leaving the flash chamber has a quality of 0.97 and the temperature in the condenser is 32oC. For 350 kw of refrigeration How much vapor must the steam ejector remove from the flash chamber?arrow_forwardA vacuum refrigeration system consists of a large insulated flash chamber kept at low pressure by steam ejector which pumps vapor to a condenser. Condensate is removed by condensate to an air vent. Warm return water enters the flash chamber at 13oC, chilled water comes out of the flash chamber at 5oC Vapor leaving the flash chamber has a quality of 0.97 and the temperature in the condenser is 32oC. For 350 kw of refrigeration How much make-up water is needed?arrow_forward
- A vacuum refrigeration system consists of a large insulated flash chamber kept at low pressure by steam ejector which pumps vapor to a condenser. Condensate is removed by condensate to an air vent. Warm return water enters the flash chamber at 13oC, chilled water comes out of the flash chamber at 5oC Vapor leaving the flash chamber has a quality of 0.97 and the temperature in the condenser is 32oC. For 350 kw of refrigeration A) How much chilled water at 5oC does this system provide? B) How much make-up water is needed? C) How much vapor must the steam ejector remove from the flash chamber?arrow_forwardAn air conditioner with refrigerant-134a as the refrigerant is used to keep a large space at 20°C by rejecting the waste heat to the outside air at 37 °C. The room is gaining heat through the walls and the windows at a rate of 125 kJ/min while the heat generated by the computer, TV, and lights amounts to 0.7 kW. Unknown amount of heat is also generated by the people in the room. The condenser and evaporator pressures are 1200 and 500 kPa, respectively. The refrigerant is saturated liguid at the condenser exit and saturated vapor at the compressor inlet. If the refrigerant enters the compressor at a rate of 65 L/min and the isentropic efficiency of the compressor is 70%, determine (a) the temperature of the refrigerant at the compressor exit, (b) the rate of heat generated by the people in the room, (c) the COP of the air conditioner, and (d) the minimum volume flow rate of the refrigerant at the compressor inlet for the same compressor inlet and exit conditions.arrow_forwardA cooling system with a capacity of 10 tons of refrigeration operates at 210 KPA in the evaporator while in the condenser it is 850 KPA if the R-134a refrigerant is in a saturated state calculate the theoretical power required to operate the compressor.arrow_forward
- Refrigerant-134a enters the condenser of a residential heat pump at 800 kPa and 35°C at a rate of 0.018 kg/s and leaves at 800 kPa as a saturated liquid. If the compressor consumes 1.2 kW of power, determine the COP of the heat pump?arrow_forwardRefrigerant-134a enters the compressor of a refrigerator as superheated vapor at 0.14 MPa and -10oC at rate of 0.05 kg/sec and leave at 0.8 MPa and 50oC. The refrigerant is cooled in the condenser to 26oC and 0.72MPa and is throttled to 0.15MPa. Determine (a) the rate of heat removal from the refrigerated space and the power input to the compressor, (b) the isentropic efficiency of the compressor and (c) the coefficient of performance.arrow_forwardAmmonia refrigerant circulates in a refrigeration system with one compressor serving two evaporators where one evaporator carries a load of 35 kW at 10°C and the other a load of 70 kW at -5°C. A back pressure valve reduces the pressure in the 10°C evaporator to that of the -5°C evaporator. The condensing temperature is 37°C. Calculate the mass of the refrigerant in kg/s. (Use h₂ = h1 + 203.7586 kJ/kg)arrow_forward
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





