
Concept explainers
Ten mL of pure liquid water in a cylinder with a movable piston is heated at a constant pressure of 1 atm from an initial temperature of 80°C. The temperature of the system is monitored, and the following behavior is observed:
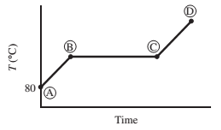
(a) What is happening in steps AB, BC, and CD? What is the temperature corresponding to the horizontal portion of the curve?
(b) Estimate the volume occupied by the water at points B and C. (Assume the vapor follows the ideal-gas equation of state.)
(a)
Interpretation:
Identify the process in AB, BC, CD areas and calculate horizontal zone temperature.
Concept introduction:
Energy is transferred into a system when it is heated. The system will change depending on the energy it receives. This can happen through increase in temperature. A heating curve is called a plot of the temperature versus time.
Answer to Problem 6.1P
- AB − Water liquid heating zone (temperature increase)
- BC − Vaporization of water (liquid to gas phase)
- CD − water vapor (gas phase) heating zone
Horizontal portion temperature = 100°C
Explanation of Solution
AB Step
As heat is absorbed, the temperature of the liquid begins to increase this is due to the increase in the kinetic energy of the molecules of liquid. For standard atmospheric pressure, the rise in temperature takes place until it reaches to 100°C. With increasing temperature, the volume of the liquid remains constant due to little expansion for liquids.
BC Step
At this point, the heat is consumed to begin vaporization of liquid. This temperature is known as the bubble point temperature. Due to change in the previous state, the temperature remains constant and water molecules are moving from liquid to vapor phase. At point C, the last drop of liquid gets evaporated.
CD Step
The temperature increases above 100 °C, when heating steam boils all the liquid into vapor. This cause increase in the volume. Step B shows transition of liquid to vapor. The boiling point of water is 100 °C (liquid here is water) and pressure is 1 atm.
(b)
Interpretation:
Volume occupied by water at point B and C should be calculated.
Concept introduction:
The ideal gas equation is represented as follows:
Here,
Answer to Problem 6.1P
At point B, the water is present as liquid only thus, the volume will be 10 mL
At point C
Explanation of Solution
At point B, the water is present as liquid only thus, the volume will be 10 mL
The number of moles in 10 mL of liquid water is calculated as follows:
The volume of vapor can be calculated using the ideal gas equation as follows:
Here, number of moles is 0.555 mol at 1 atm and
Putting the values,
Thus, at point C, the volume occupied by the water occupies is 17 L.
At point C,
Want to see more full solutions like this?
Chapter 6 Solutions
Elementary Principles of Chemical Processes
Additional Engineering Textbook Solutions
Elements of Chemical Reaction Engineering (5th Edition) (Prentice Hall International Series in the Physical and Chemical Engineering Sciences)
Process Dynamics and Control, 4e
Experiencing MIS
Starting Out with C++: Early Objects
Starting Out with Java: Early Objects (6th Edition)
Starting Out With Visual Basic (8th Edition)
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The





