
Concept explainers
Assign the peaks in the
NMR spectrum of eugenol (Fig. 6.23) to specific protons in the molecule. The OH peak is at 5.1 ppm.
Interpretation:
The peak in
Concept introduction:
NMR is an analytical method that is utilized to identify the structure of a compound. It determines the framework of carbon-hydrogen bonds present in the compound.
Number of signals in the
The protons that are present in the different chemical environment are known as non-equivalent protons while the protons that are present in same chemical environment are known as equivalent protons.
Answer to Problem 1Q
Eugenol has 12 different hydrogen atoms. The three hydrogen atom present on the aromatic ring show peak around
Explanation of Solution
The structure of eugenol is as follows:
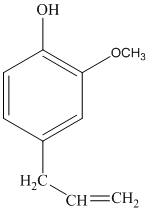
Eugenol has 12 different hydrogen atoms. The integration of peaks from right to left is 3:2:2:3:2.
There are two peaks for the three hydrogens around
There are two peaks for four hydrogen atoms around
There are two peaks for six around
Want to see more full solutions like this?
Chapter 6 Solutions
Macroscale and Microscale Organic Experiments
- Definition and classification of boranes.arrow_forwardWhich of the terms explain the relationship between the two compounds? CH2OH Он Он Он Он α-D-galactose anomers enantiomers diastereomers epimers CH2OH ОН O он Он ОН B-D-galactosearrow_forwardHi, I need help on my practice final, If you could offer strategies and dumb it down for me with an explanation on how to solve that would be amazing and beneficial.arrow_forward
- Hi I need help with my practice final, it would be really helpful to offer strategies on how to solve it, dumb it down, and a detailed explanation on how to approach future similar problems like this. The devil is in the details and this would be extremely helpfularrow_forwardIn alpha-NbI4, Nb4+ should have the d1 configuration (bond with paired electrons: paramagnetic). Please comment.arrow_forwardHi, I need help on my practice final, if you could explain how to solve it offer strategies and dumb it down that would be amazing. Detail helpsarrow_forward
 Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
 Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning





