
Concept explainers
Determine the number of nodes along the internuclearaxis for each of the
Interpretation: The number of nodes along the internuclear axis for each of the
Concept introduction: Born-Oppenheimer approximation state that nuclei are heavier than electrons that considered fixed in space whereas the electrons move constantly around them. The Born−Oppenheimer approximation solves the electronic Schrodinger equation for
Answer to Problem 1P
The number of nodes in
Explanation of Solution
Node is point in molecular orbitals where probability to find the electron density is zero. Nodal plane is the imaginary plane where probability to find electrons is zero.
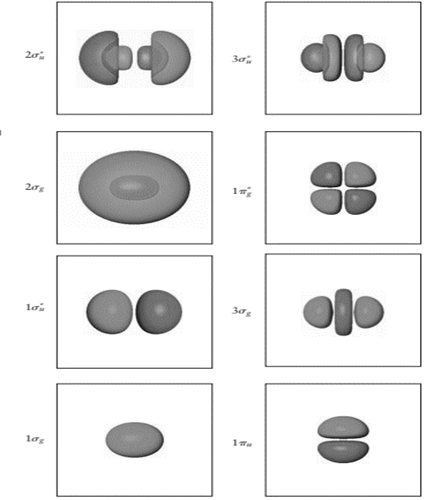
In the figure, node is the oval region. It is formed when plane white surface cut the spherical orbital into two equal halves having opposite symmetry. Also, there are six
In
In
In
In
In
In
Therefore, number of nodes in
Want to see more full solutions like this?
Chapter 6 Solutions
Principles of Modern Chemistry
- Use MO theory to predict the bond order and the number of unpaired electrons in the super-oxide ion, , and the peroxide ion, .arrow_forwardDetermine if the following species have permanent dipole moments. a Dichloromethane, CH2Cl2 b Chlorobenzene, C6H5Cl c Ammonia, NH3 d Carbon dioxide, CO2.arrow_forwardDetermine if the following species have permanent dipole moments. a The carbonate ion, CO32 b The phosphate ion, PO43 c Uranium hexafluoride, UF6 d Bromine, Br2.arrow_forward
- Why does the energy ordering of the molecular orbitals of the period 2 diatomic molecules change in going from N2 to 0,?arrow_forwardThis molecule has [x overlapping atomic p orbitals that combine to make [y]. molecular pi orbitals.arrow_forwardHow many molecular orbitals are generated from the linear combination of two sp2 orbitals?arrow_forward
- Please explain.arrow_forwardConstruct the molecular orbital diagram showing the bonding and antibonding molecular orbitals from combinations of two s orbitals using H2 as an example [Write the generic wave functions of the molecular orbitals]arrow_forwardFor the first ionization energy for an N2 molecule, what molecular orbital is the electron removed from?arrow_forward
- In the formation of CO, the 2s orbital from C and 2p orbital from O could also participate in the formation of sigma molecular orbital. Explain why this is possiblearrow_forwardIn the formation of CO, the 2s orbital from C and 2p orbital from O could also participate in the formation of σ molecular orbital. Explain why this is possiblearrow_forwardWhen considering molecular orbital diagrams of homonuclear diatomic molecules, explain why atomic orbital energies are the same. How will this differ for heteronuclear diatomic molecules?arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,
Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning, Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





