
Concept explainers
(a)
To determine: The value of Vmax and Km of enzyme prostaglandin endoperoxide synthase.
Introduction:
Prostaglandin is class of lipid which is present at a site of injury and tissue damage in body. It is involved in healing process and induces inflammation, and initiation of pain.
(a)
Explanation of Solution
Pictorial representation:
Table 1 shows the rate of formation of prostaglandin from arachidonic acid and Fig.1 shows the Lineweaver Burk plot, a double reciprocal plotting for 1/V0 Vs. 1/[S].
Table 1
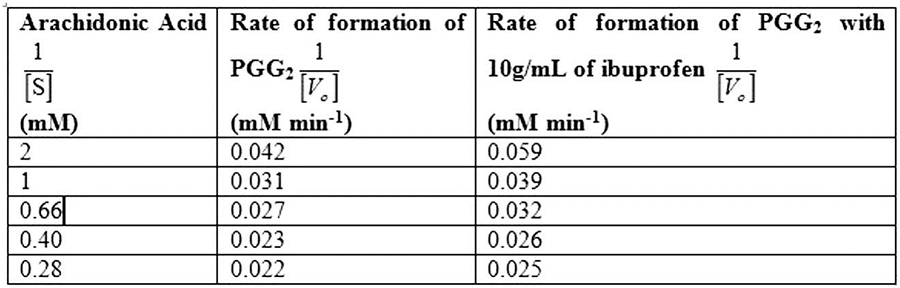
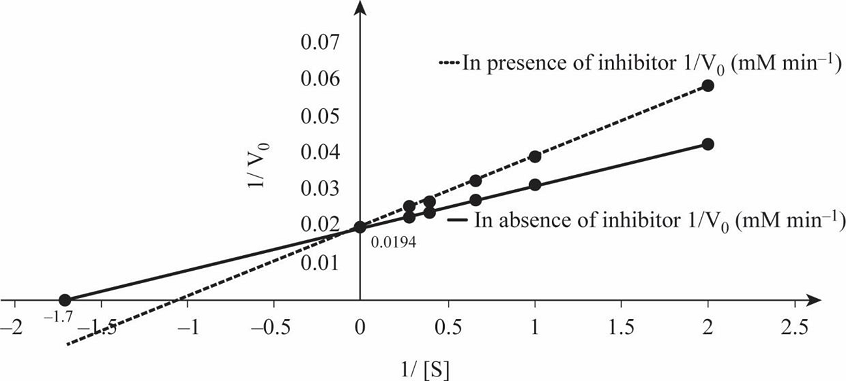
Fig.1: Lineweaver Burk plot.
Lineweaver-Burk equation is the reciprocal of Michaelis-Menten equation, and given as:
Michaelis-Menten equation
Lineweaver-Burk equation
So, by reciprocating the values given in first and second columns of the given table, we get 1/[S] and 1/V0 values in absence of the inhibitor, as mentioned in Table 1. By plotting these values we obtain the Lineweaver-Burk graph depicted in Fig.1. From the graph, calculating the V max in the absence of inhibitor:
Calculating the Km in the absence of inhibitor:
The Vmax of enzyme in the absence of inhibitor is 51.5mM/min, while Km of enzyme in the absence of inhibitor is 0.59mM.
(b)
To determine: The type of inhibition that ibuprofen exerts on prostaglandin endoperoxide synthase.
Introduction:
Enzyme inhibitors are defined as chemical molecules that bind at active site of enzymes and prevent the binding of substrate with enzyme. There are two types of inhibitors such as reversible and irreversible inhibitors.
(b)
Explanation of Solution
Lineweaver-Burk equation is the reciprocal of Michaelis-Menten equation is given as:
Michaelis-Menten equation
Lineweaver-Burk equation
So, by reciprocating the given values in column first and third, we get the rate of formation of prostaglandin from arachidonic acid in presence of inhibitor ibuprofen, as mentioned in Table 1. By plotting these values we obtain the Lineweaver-Burk graph for 1/[S] and 1/V0 in presence of inhibitor, as depicted in Fig.1.
Calculating the Vmax in presence of inhibitor:
Vmax in the presence of inhibitor:
Km of enzyme in the presence of inhibitor:
The Vmax of enzyme both in the presence and absence of inhibitor is 51.54 mM/min, while Km of enzyme in the presence and absence of inhibitor is 0.83mM and 0.59mM.
Prostaglandin is involved in initiation of pain, and it is synthesized by prostaglandin endoperoxide synthase. Ibuprofen inhibits the activity of this enzyme by binding at the active site and preventing binding of substrate with enzyme. The double reciprocal graph in Fig.1 shows when competitive inhibitor ibuprofen is present, the Vmax remains unchanged while Km increases. Thus, -1/Km value is closer to the origin in the graph depicted in Fig.1. In competitive inhibition, Vmax remains unchanged while Km increases. Therefore, ibuprofen is a competitive inhibitor of prostaglandin.
The inhibition of prostaglandin by ibuprofen is example of competitive inhibition.
Want to see more full solutions like this?
Chapter 6 Solutions
EBK LEHNINGER PRINCIPLES OF BIOCHEMISTR
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





